Biochemistry of the vision process
Visible light is electromagnetic radiation (400–750 nm). It spreads through the environment, refracts and absorbs in different spectra. It reaches the retina through the optical environment of the eye(cornea - chamber water - lens - vitreous). The photoreceptors of the retina convert light energy into atomic motion, the chemical change translating into a nerve impulse propagating to the brain.
Photoreceptor cells of the retina[edit | edit source]
The retina is composed of interconnected nerve cells, whose arrangement allows histological differentiation of 10 layers, light passing through the upper layers to the layer of two types of luminous cells - rods and cones. Their distribution in the retina is not uniform. Their function can be impaired by, among other things, retinal detachment.
Rods[edit | edit source]
They provide vision even in low light intensity - scotopic vision. But they don't detect colours. In terms of the number of lens cells, they make up the vast majority - 130 million. They are more concentrated in the peripheral parts of the retina. The inner segment' is highly metabolically active, producing abundant ATP and protein. The outer segment' forms densely stacked discs. Their membrane contains the chromophore RHODOPSIN (so-called visual purple), a spectrum-dependent pigment.
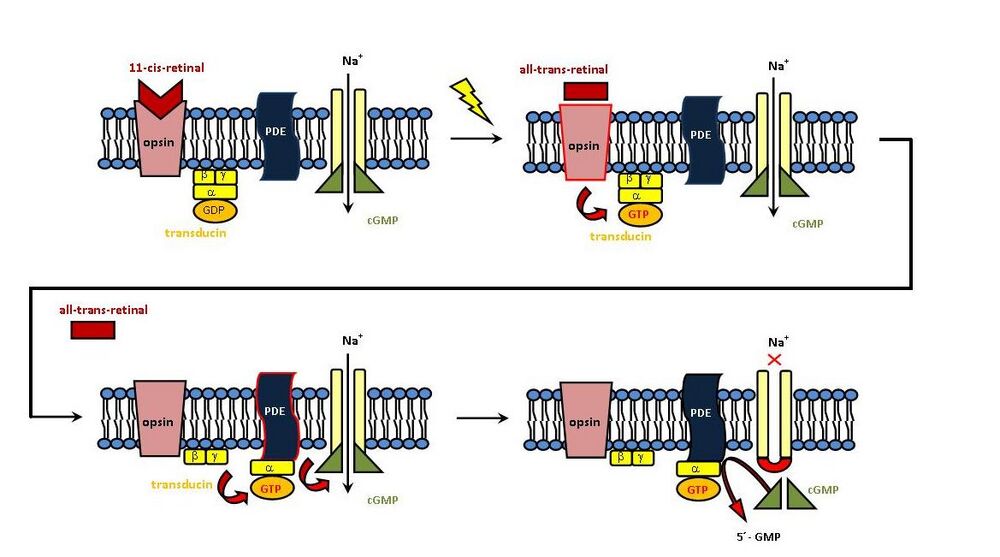
- Rhodopsin' - a covalently bound complex of the protein OPSIN' and 11-cis-RETINAL (a derivative of vitamin A). The complex is formed by the reaction of the aldehyde group of retinal with the NH2 group of the lysine residue of the opsin molecule (= Schiff base).
- 'Opsin - a protein in the membrane of the disc of the outer segment of the rod, composed of 7 helices = 7 times passing through the membrane, protruding on both sides.
- '11-cis-retinal' - low molecular mass dye, binds roughly in the middle of the membrane between the helices of opsin.
In the dark'' the outer segment is strongly penetrated by Na+ ions' through specific membrane channels, the sodium pump of the inner segment (Na+, K+ ATPase) maintains a high concentration gradient (the resulting potential is about -40 mV). The Na+ channel is kept open by cyclic guanosine monophosphate (cGMP).
Cones[edit | edit source]
They provide vision in good light conditions - photopic vision. They perceive colours. There are almost 20 times fewer of them than rods - 7 million. Their greatest concentration is in the yellow spots. There are several theories explaining their colour sensitivity. At present, perhaps the most widely accepted distinction is that of cones into 3 types according to their sensitivity to wavelength. They contain photopigments with different absorption maxima.
The chemical basis of rod vision[edit | edit source]
The process of vision consists of a cascade of chemical reactions from the impact of a photon to the generation and transmission of excitation.
When light hits the retina, it is absorbed. The rods are incredibly sensitive, reacting to the impact of a single photon. Absorption leads to excitation of the membrane, which results in isomerization of 11-cis-retinal to ALL-TRANS-RETINAL. The energy of the photon is thus transformed into atomic motion.
The light activation is very fast and yet complex. Within milliseconds, a series of photochemical reactions take place whose intermediates (bathorhodopsin, lumirhodopsin, metarhodopsin I, metarhodopsin II) show different maxima from 500 to 380 nm.
The following consequence of the photon impact is uncoupling of the dye from the protein. The trans-isomer no longer fits into the binding site. Rhodopsin thus breaks down into opsin and all-trans-retinal. This activated rhodopsin further activates the G-protein TRANSDUCIN. The cascade continues with the activation of PHOSPHODIESTERASE (PDE), which hydrolyzes cGMP to NON-cyclic form (5´-GMP). The originally open channel for Na+ ions closes, the ion flow stops. The consequence is HYPERPOLARIZATION of the membrane, it becomes more negative (hyperpolarization is only -35 mV here, because the resting membrane potential is -30 mV ). Hyperpolarization spreads to the synapse, allowing the transmission of excitations further along the visual pathway. From the ganglion cells, the signal continues as depolarization. The value of hyperpolarization depends on the intensity of illumination. In addition, the signal sent from a single photon is greatly amplified by hyperpolarization.
In the dark', the trans isomer is converted back to 11-cis-retinal (retinal isomerase), the opsin+11-cis-retinal complex is formed again, and the process is repeated after further irradiation = Wald cycle.
However, this cyclical process is complicated by reactions outside the retina. The released trans-retinal is partly carried by the blood to the liver, where it is hydrogenated to the alcohol TRANS-RETINOL and isomerized to CIS-RETINOL, the latter being carried by the blood back to the retina, where it must be oxidized to 11-cis-retinal. Blood transfer is enabled by binding to the transport protein retinol-binding protein (RBP).
As the preceding text shows, a sufficient amount of vitamin A (retinol) and its provitamin β-carotene are required for proper retinal function.
The chemical basis of cone vision[edit | edit source]
In the colour vision process using cones, the reactions after the photons hit are pretty much the same as in rods. Activated rhodopsin in cones activates transducin, phosphodiesterase converts cGMP to 5'-GMP, Na+ channels are closed - hyperpolarization is induced. Colour vision is determined by the presence of three types of cones. The chromophore is the same as in the rods - 11-cis-retinal capable of isomerization. However, their photoreceptors have a slightly different protein (photopsin), causing differences in absorption'. They absorb wavelengths of light with different maxima:
- blue 440 nm;
- green 535 nm;
- red/yellow 565 nm.
The perception of the other colours should be based on the combination and intensity of the irritation of these three types of cones.
Adaptation[edit | edit source]
Dark adaptation[edit | edit source]
During the transition from light to darkness, the function of the retina changes, going from photopic to scotopic vision - increasing sensitivity to light, sensitized by synthesis (increase in concentration) of rhodopsin. It takes tens of minutes to hours for the process to get into full effect. Therefore, vision is decreased with a sudden reduction in light intensity. Perfect adaptation takes up to 60 minutes. Sufficient vitamin A is required.
The function of the cones is suppressed, we stop seeing colors. The maximum spectral sensitivity of the eye shifts from the 550 nm region to a shorter wavelength of around 505 nm during adaptation, this wavelength corresponds to the maximum spectral sensitivity of rhodopsin. Thus, the colours of the short-wavelength spectrum appear brighter in the dark than the long-wavelength colours - the Purkinje effect.
Light adaptation[edit | edit source]
When returning from darkness to light, the rods have to be switched off, but the cones adapt relatively quickly, the eye adjusts to the intense light within 3 minutes.
Retinal photoreceptor disorders[edit | edit source]
Retinitis pigmentosa - mutation of the gene for rhodopsin, inherited, light-harvesting cells die, leading to blindness.
Avitaminosis A - not only limits rhodopsin regeneration - glaucoma, causes morphological changes to destruction of receptors.
Daltonism - deficiency of photoreceptor protein absorbing green or red, common (1-2% of population).
Protanopia - impaired vision of red colour.
Deuteranopia - impaired green vision.
Tritanopia - blue vision impairment.
Sources[edit | edit source]
Related articles[edit | edit source]
Bibliography[edit | edit source]
- AUTRATA, Rudolf – VANČUROVÁ, Jana. Nauka o zraku. 1. edition. Institut pro další vzdělávání pracovníků ve zdravotnictví, 2002. pp. 226. ISBN 80-7013-362-7.
- LEDVINA, Miroslav – STOKLASOVÁ, Alena – CERMAN, Jaroslav. Biochemie pro studující medicíny : II. díl. 1. edition. Karolinum, 2004. ISBN 80-246-0850-2.