Redox potential
(Redirected from Redox potencial)
Oxidation-reduction potential (redox potential) is a measurement of the ability of substances to bind or release electrons , i.e. is a measurement of the strength of an oxidizing or reducing agent.
Electron affinity can be expressed as the potential to which an electrode immersed in a solution containing oxidized and reduced form of the same substance is charged - we call this system a half -cell .
As soon as we dip an electrode made of a noble metal (platinum or gold wire) into a solution containing equal amounts of the reduced and oxidized form of the same substance, this electrode is charged to a certain potential due to the solution.
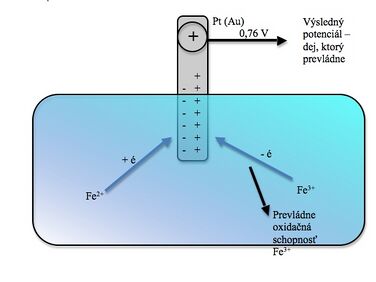
- Example
We note two conflicting events:
- ferrous ions tend to donate electrons to the electrode and create an excess of negative charge on it, they are oxidized to ferrous ions,
- ferrous ions try to take electrons from the electrode and thereby reduce themselves to ferrous, thus creating an excess of positive charge on it.
The resulting potential to which the electrode is charged depends on which of the events prevails , in our case the oxidizing ability of ferric ions prevails and the electrode is charged positively to a value of 0.76 V (at the same eastern concentration of both ions).
Measurement of redox potential[edit | edit source]
We are not able to measure absolute values of redox potentials, so we compare them with so-called reference electrodes - they have a known and constant potential.
- both electrodes (reference and the one whose potential we want to measure) are immersed in the same solution and connected by a so-called salt bridge (most often a KCl solution) - this creates a galvanic cell .
An international agreement establishes a system of hydrogen, hydrogen cation and a platinum electrode as the basis for measuring redox potentials . The redox potential of this group is, by agreement, zero and serves as the basis of the scale of redox potentials.
Links[edit | edit source]
Related articles[edit | edit source]
External links[edit | edit source]
References[edit | edit source]
- STOKLASOVÁ, Alena – KŘÍŽALA, Josef – ŠIMAN, Pavel. Obecná, fyzikální a anorganická chemie pri studující medicíny. 1. edition. Praha : Karolinum, 1996. vol. 1. pp. 91. ISBN 80-7066-949-7.