Major Histocompatibility Complex
(Redirected from Major histocompatibility complex)
Major histocompatibility complex is in humans called human leukocyte antigen (HLA). In humans the MHC genes are located on a short arm of chromosome 6.
MHC genes are highly polymorphic. For each gene there are many variants of its alleles. On the chromosome is located a specific combination of allels called haplotype. Each person has two haplotypes inherited from parents. Because of a very tight linkage, recombinations are not very frequent.
Structure of MHC[edit | edit source]
There are three groups of MHC molecules: class I (HLA-A,B,C), class II (HLA-D), class III. First two groups are derived from the same common gene and are very similar to each other.
The third class is different. Its locus is between the other two classes but this position is a result of translocation during evolution. Proteins of MHC have similar structure to imunoglubulins.
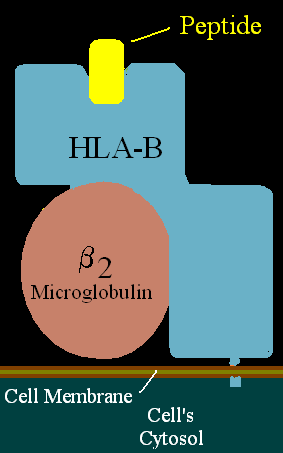
Class I[edit | edit source]
Proteins of this class can be found on all nucleated cells. It consists of α chains and β2 microglubulin molecule. α-chains (α1, α2, α3) are glycoproteins with three units: external, transmembrane and cytoplasmatic. α1 and α2 create together a heterodimer and make the place where peptide is held. β2 microglubulin molecule is located below the α1 unit.
Peptides which are displayed on a cell surface are mostly self peptides. These come from proteins which have been degraded in proteasome.
In case of viral infection or cancerous transformation these proteins are transformed and displayed. Thank to this process, the T- lymfocytes (cytotoxic T-lymfocytes) can detect transformations and react to them.
Class II[edit | edit source]
Proteins of this class occur on B- lymfocytes, dendritic cells, monocytes, macrophages. It consists of two chains, α and β. Both have four units.
External, connecting, transmembrane, cytoplasmatic unit. The external unit has two subunits. Variable and constant. Variable subunit consists of α1 and β1 and constant subunit is represented by α2 and β2. The place for peptide is created by a heterodimer of α1 and β1.
Peptides which are displayed on cell surface come from phagocytosis. Phagocyted molecules are processed in endosomes and then displayed on the cell surface so that T-lymfocytes (helper T-lymfocytes) can recognize them.
Class III[edit | edit source]
This class is different from the former two. Molecules of this class include several proteins with immune functions, such as: cytokines, heat shock proteins, parts of complement system.
Function[edit | edit source]
Basic function of major histocompatibility complex (MHC) is to display peptidic fragments (epitopes or antigenic determinants) of protein molecules, which has been produced or swallowed by a cell. These antigenic determinants can be found on the cell surface and afterwards presented to T-lymfocytes.
T-lymfocytes are able to recognize whether the antigenic determinant is self or non-self. If a non-self moiety is identified an immune reaction starts. This can cause problems in transplantations. The transplanted tissue can be recognized as a non-self (because of a different MHC proteins on a cell surface) and an immune reaction is triggered.
Bibliography[edit | edit source]
HOŘEJŠÍ, BARTŮŇKOVÁ,. Základy imunologie. 4. edition. 2009. ISBN 978-80-7387-280-9.