X-ray radiation - mechanism of formation
X-rays are electromagnetic waves characterized by short wavelengths and high energy. An X-ray machine serves as the basic source of X-ray radiation (previously, ion lamps were used). The basis of the mechanism of X-ray generation is the interaction of electrons released by thermoemission from the cathode with atoms of the anode. When electrons hit the anode, over 99% of their kinetic energy is converted into heat, the remaining energy is converted into X-rays. X-ray radiation is produced in the X-ray machine in two ways – as bremsstrahlung and characteristic radiation.
Construction and function of the X-ray[edit | edit source]
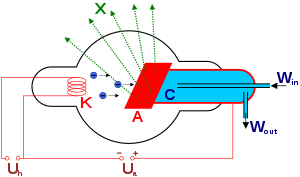
It is an evacuated glass tube, the so-called Coolidge lamp, in which the electrodes - cathode and anode - are built. The incandescent tungsten cathode is shaped like a spiral and is placed in a negatively charged molybdenum cup. The main function of this dish is to repel and concentrate (focus) the released electrons on the surface on the anode. The anode is usually also made of tungsten, which is characterized by a high atomic number, high melting point and good thermal conductivity. The high melting point prevents the anode from melting, the thermal conductivity ensures the removal of heat generated during the impact of electrons. We can encounter a molybdenum anode during mammography.
The cathode is heated from a glow transformer. Electrons are released by thermoemission from the cathode and form a cloud of varying density. Due to the high voltage inserted between the electrodes (tens to hundreds of kV), the electrons are attracted and accelerated to the anode. They fall sharply on the so-called impact focus, which is inclined and makes an angle of approximately 19° with the exit window of the X-ray machine. Thanks to this inclination, the surface on which the electrons fall has the shape of a narrow rectangle and is called the thermal focus. By projecting the thermal focus in the direction of the central beam, the focus is optical. The sharpness of the X-ray image depends on the size of the optical focus. The smaller the optical focus, the sharper the image. However, with the sharpness of the image, the load capacity of the X-ray tube decreases. (The load capacity indicates the power that the X-ray can withstand for one second.) That is why the so-called rotating anodes formed by a rotating tungsten disk. There is an increase in the thermal focus. The load capacity then depends on the diameter of the anode and the speed of its rotation.
When electrons hit the anode, a large amount of heat is generated, so the anode must be cooled. We distinguish between air and oil cooling, which is sometimes combined with water cooling.
The X-ray machine is mechanically protected by a metal cover, which also provides a number of other functions (connection of high-voltage cables, fixing of the primary screen, protection of workers from high voltage (thanks to grounding) and from ionizing radiation ). There is still a layer of insulating oil between the cover and the X-ray tube. A beryllium plate is placed in the cover of the X-ray machine - the exit window. Electrons cannot pass through the plate, only usable X-rays.
The penetration of X-rays depends on the wavelength, and therefore on the voltage between the electrodes (the higher the voltage, the shorter the wavelength), the intensity of the rays depends on the number of released electrons, and therefore on the temperature of the heated filament of the cathode.
Bremsstrahlung[edit | edit source]
When a fast-flying electron enters the electrostatic field of the nucleus of the anode atoms, an electromagnetic interaction occurs that causes the path to curve and the electron's speed to decrease sharply. Braking an electron causes the electron to lose some of its kinetic energy. This energy is converted into a photon of X-ray radiation. X-ray photons have different wavelengths and carry different energies, bremsstrahlung therefore has a continuous spectrum. As the energy of the electron increases, the frequency of the generated X-ray radiation increases, while the wavelength decreases. The quality of the braking radiation depends only on the anode voltage.
Characteristic X-rays[edit | edit source]
Characteristic X-ray radiation is produced differently than bremsstrahlung radiation. If the electrons falling on the anode have sufficient energy, this energy is transferred to the electrons of the inner shell of the electron shell, which results in their displacement (excitation) to higher energy levels or, more often, complete ejection from the atom ( ionization ). Electrons from higher energy levels of the atom (or even free electrons) then jump to the vacant place, whereby the atom tries to regain its stability. Since there are significant energy differences between the individual energy levels, the energy difference is emitted in the form of a photon of electromagnetic radiation, or characteristic X-ray radiation.
The atom of each element (in this case a metal - e.g. vanadium, copper) has its characteristic values of energy levels of electrons in the shell. Each element therefore has a typical spectrum, referred to as a line spectrum for its discrete energy, by which it can be identified. So we are talking about radiation that is characteristic of the material from which the anode is made. The share of characteristic X-ray radiation in the total spectrum of X-rays depends on the anode voltage. If the voltage on the X-ray tube is gradually increased, X-rays with a continuous spectrum are emitted at low voltage values. If this voltage begins to rise, both types of X-rays are produced by the X-ray and their spectra are stacked.
Links[edit | edit source]
Related Articles[edit | edit source]
References[edit | edit source]
- NAVRÁTIL, Leoš – ROSINA, Jozef. Medicínská biofyzika. 1. edition. Grada, 2005. 524 pp. ISBN 80-247-1152-4.
- BENEŠ, Jiří. Základy lékařské biofyziky. 1. edition. Karolinum, 2005. 196 pp. ISBN 80-246-1009-4.