Radioactivity Practical (Group 4)
|
Check of this article is requested. Suggested reviewer: Carmeljcaruana |
Radioactivity [edit | edit source]
Group 4B[edit | edit source]
Introduction: [edit | edit source]
Radioactivity or radioactive decay can be identified by the emission of particles from unstable atomic nuclei. Unstable atomic nuclei are ones that do not possess enough binding energy to hold the nucleus together due to an excess of either protons or neutrons.
Radioactive decay can be worked out using quantum physics, but it is still inherently probabilistic, making it impossible work out when an atom will decay. However, predictions can be made based on the statistical behaviour of many atoms. This predictive measure of radioactive probability is called the half-life of an atom. It is defined as the time after which, on average, half of the original material will have decayed.
There are several different types of radiation that are currently known with a wide range of uses both inside and outside of medicine. The type of radiation with the widest array of clinical application is ionising radiation, example of these are X-rays, gamma radiation and highly electromagnetic ultraviolet light. Ionising radiation is radiation with enough energy to cause a change in charge of an atom, induce radioactive decay and, therefore alter how an atom or molecule functions.
Importance in clinical medicine:[edit | edit source]
A vital use of radiation in medicine is the use of radiotracers (or tracers). Tracers are small amounts of either beta or gamma emitters that are inserted into the bloodstream. Tracers are used to investigate and track certain functions in the patient’s body for the purpose of diagnosis. PET (positron emission tomography) also uses radiotracers to track biochemical reactions in the body.
In oncology, radiation is important for diagnosis, prevention and treatment. PET scans are used to locate tumours and diagnose cancer and regular screening can help prevent cancer. Gamma radiation is used in medicine to sterilize medical equipment and prevent bacterial infection in patients as well as the treatment of cancer by targeting and killing cancerous cells and is known as radiotherapy (RT). As vital as radiation is to diagnosis, frequent and prolonged use can cause cancer (due to alteration of DNA sequence in cells) which is why its recommended for physicians to only use radiation when necessary and to refer the patient to the risks of the radiation before exposing them to it.
Brief literature review:[edit | edit source]
Advantages and Disadvantages of Nuclear Medicine:[edit | edit source]
- The high precision of the instruments used allows for a simpler and less invasive methods of treating the patient.
- Nuclear medicine is less expensive and may yield more precise information than exploratory surgery.
- PET scans can identify lesions as either benign or malignant, thus removing the need for a surgical biopsy or determining the optimal biopsy location.
- Although very rare and mild, allergic reactions to radiopharmaceuticals may occur in patients.
- There is a risk that the patient`s healthy cells
may be affected by the radiation used during diagnostic imaging. This risk is highest in patients that are young, pregnant or elderly.
- Due to the scattering of the ionizing radiation and its unpredictability, the radiologists are under a certain risk of absorbing some of the emitted
radiation and are required to wear protective clothing.
How does radioactivity work? [edit | edit source]
Some nuclei are unstable, and this instability leads to a rearrangement of the protons and neutrons which results in the emission of radiation (radioactivity).
Three main types of radiation:[edit | edit source]
- Alpha decay: A process which results in the
emission of an alpha particle (2 protons and 2 neutrons) when there are too many protons in the nucleus of an atom.
- Beta decay: A process which results in the
emission of either a positive or a negative beta particle, due to an imprope protons-neutron ratio inside the nucleus of an atom.
- Gamma decay: A process which results in the emission of energy, due to the returning of the protons or neutrons to their right position on the energy level in thenucleus of an atom.
Methods used in nuclear medicine:[edit | edit source]
PET (Positron Emission Tomography): PET scans measure metabolic activity and molecular function by using a radioactive glucose injection. When injected into the patient, the PET scanner detects the radiation emitted from the patient, and the computer generates 3D images of tissue or cell activity in the tissues of the body.
Radioactive Iodine: The use of radioactive iodine also known as ( I-131) to treat an overactive thyroid, a condition called hyperthyroidism. When a small amount of I-131 is swallowed, it is absorbed into the bloodstream in the gastrointestinal (GI) tract and concentrated from the blood by the thyroid gland, where it begins destroying the gland’s cells.
Other examples: Gamma camera, etc.
Ethical problems:[edit | edit source]
With every ionizing radiation image taken, carries a cancer risk for the patient, so we always have to think about the benefits and the risks of the patient before proceeding with any examination. When examined, the results must be in sufficient quality in order to have accurate diagnosis and avoiding any overdose which can be a high risk for the patient if not needed.
Equipment:[edit | edit source]
- Excel spreadsheet
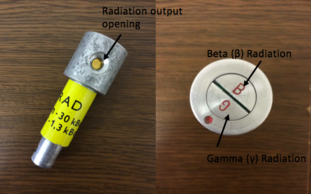
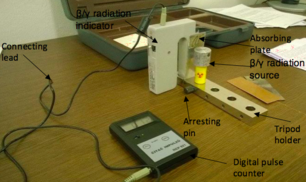
- GamaBeta kit:
1. Radiation indicator
2. Beta and Gamma radiation source
3. Absorbing plates:
Aluminium
Iron
Copper
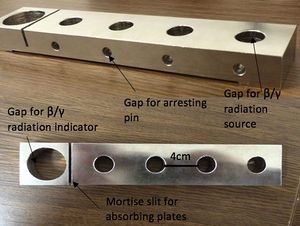
4. Arresting pin
5. Tripod holder
6. Digital pulse counter
[edit | edit source]
[edit | edit source]
Methodology:[edit | edit source]
- Set up the GamaBeta kit by placing the Beta/Gamma radiation indicator on the tripod holder
- Connect the digital pulse counter to the β/γ radiation indicator by using the connecting lead and turn on the sliding switch for both appliances
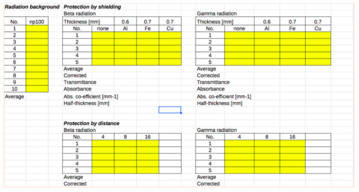
- In order to assess the background radiation of the environment that you will be working in, make sure that there are no radiactive materials nearby as this will interfere with the measurements recorded, therefore place the radioactive source in another room
- Measure the background radiation for 10 seconds by pressing the ‘100s’ button on the digital pulse counter and divide the results by 10
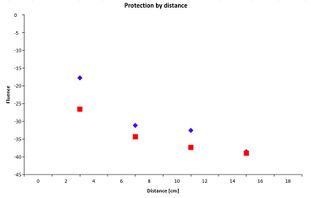
- For protection of the radiation by distance, place the radiation source 4cm from the β/γ radiation indicator (first gap closest to the indicator). To sense the beta particles, make sure that the opening mouth of the radiation output is facing the side where it has ‘B’ marked at the head of the source. Correspondingly, to recognise the gamma particles, make sure the opening is on the side where it has ‘G’ marked on the head
- Insert the arresting pin into the gap on the tripod holder perpendicular to the radiation source
- Measure for 10 seconds by pressing the ‘10s’ button on the pulse counter and repeat 5 times to obtain an average value
- Repeat the procedure from steps 5 - 7 again but increase the distance of the radiation source and the arresting pin to 8 cm (second gap away from the β/γ radiation indicator) and then to 16cm (fourth gap away from the β/γ radiation indicator). Take 5 measurements at each distance
- Repeat steps 5 - 8 with gamma radiation, making sure the opening is on the side where it has ‘G’ marked on the head
- To evaluate the protection by shielding, place the radiation source 4cm away from the counter making sure that the absorption layer is placed between the two components on the mortise slit of the tripod holder
- Take 5 measurements for both beta and gamma particles and determine an average for both types of radiation
- Repeat step 10-11 for both Iron and Copper making sure that the radiation source is still 4cm away
- Enter the data collected into an Excel spreadsheet template (shown below). This will reduce human error in calculation and can automatically create graphs. This will help you to compare each type of radiation and their correlations such as their fluences against increasing distances
- CALCULATE TRANSMITTANCES AND ABSORBANCES FOR EACH MATERIAL. CALCULATE ALSO THE ATTENUATION COEFFICIENT AND HALF-VALUE-LAYER (HVL) USING THE EQUATION I = Io e-ux WHERE u IS THE ATTENUATION COEFFICIENT.
Conclusion:[edit | edit source]
There are many ways radioactive energy is harnessed for clinical practise. Nuclear medicine is a branch of medical imaging that uses, largely, ionising radioactive material to diagnose and determine the severity of, or treat a variety of diseases.
The current use of diagnostic techniques in nuclear medicine is vast. It can be seen in PET, RT, the use of radioisotopes in Biochemical analysis and in the use of gamma irradiation for sterilising medical products and equipment (world-nuclear.org, 2016).
Non-invasive predictions in Sentinel Lymph Node Biopsy to assess lymph node metastasis are also in currently being research through increased understanding of radioactive half-life (Thangarajah, F., et al., 2016).
In conclusion, the potential for the clinical application of radioactivity is great. As our understanding of radioactive mechanisms and our ability to manipulate radioactive paraments progresses, advancements in the clinical application of radioactive decay will no doubt continue to further establish itself in medicine.
References:[edit | edit source]
Radiological Society of North America (RSNA) and American College of Radiology (ACR). "General Nuclear Medicine." Nuclear Medicine, General. Radiology Info for Patients, 17 Mar. 2016. Web. 25 Dec. 2016. http://www.radiologyinfo.org/en/info.cfm?pg=gennuclear.
"What Is Radiotherapy? - Macmillan Cancer Support." WeareMacmillan. Cancer Support, 31 May 2013. Web. 25 Dec. 2016. http://www.macmillan.org.uk/information-and-support/treating/radiotherapy/radiotherapy-explained/what-is-radiotherapy.html
Radioactivity: What it is and what it does, Donald R. Wiles, https://books.google.cz/books?id=B8E69e2eMtwC&dq. https://books.google.cz/books?id=B8E69e2eMtwC&dq
Wikipedia, [https://en.wikipedia.org/wiki/Nuclear_medicine,Health Research Funding http://healthresearchfunding.org/pros-cons-nuclear-medicine,RadiologyInfo http://www.insideradiology.com.au/radiation-risk/ https://en.wikipedia.org/wiki/Nuclear_medicine, Health Research Funding http://healthresearchfunding.org/pros-cons-nuclear-medicine, RadiologyInfo
http://www.insideradiology.com.au/radiation-risk/]
World Nuclear Association.(2016). Radioisotopes in Medicine. Available: http://www.world-nuclear.org/information-library/non-power-nuclear-applications/radioisotopes-research/radioisotopes-in-medicine.aspx
Thangarajah, F., et al. (2016). Predictors of sentinel lymph node metastases in breast cancer-radioactivity and Ki-67. The Breast. 30 (1), p87–91.