Photoelectric effect
Introduction[edit | edit source]
Photoelectric effect is one of three possible interactions of γ radiation with the electron shell. Out of these three interactions has photon usually the lowest energy. It is a physical phenomenon, where electrons are ejected from matter (usually metal) due to absorption of electromagnetic radiation. Electrons emitted in this manner are then called photo electrons. Their emission is called photoelectric emission (photoemission).
History[edit | edit source]
As discoverer of photoelectric effect is regarded Heinrich Hertz, who noticed during his experiments with a spark gap generator, that sparkling UV radiation exposure facilitates the flashover, i. e. electric charge transmission between electrodes.
In 1899 Joseph John Thompson clarified the nature of photoelectric phenomenon decisively. Thompson identified electrons in the flow of negatively charged particles emitted from the metal.
The own nature of the phenomena described Albert Einstein in 1905 in detail and earned for that the Nobel Prize in Physics in 1921
Physical description[edit | edit source]
The photoelectric effect occurs, when the entire energy of photon passes on an electron in the electron shell of the absorbing material or a free electron (e.g. in metal). Part of the energy enables emission (work function Φ) of the electron from the atom, and the rest contributes to the electron's kinetic energy as a free particle (photo electron). The work function is defined as the minimum amount of energy, that is necessary to free the electron. The gamma photon perishes and its energy is taken over by the ionizing photo electron.
Einstein's photoelectric equation formulates the law of conservation of energy: .
After absorbing the energy of photon the atom is left in an excited state and returns back to the ground state after emitting the electromagnetic radiation.
The empty space left by the emitted electron is filled by another electron from a different electron shell of the atom. During this jump energy in the form of a specific radiation is being emitted. What else can also happen is the Auger effect, where the energy is transferred to another electron of a higher electron shell, which is ejected from the atom and this second ejected electron is called an Auger electron.
Photon interacts with electrons in shells K, L and M, i. e. electrons close to nucleus. The interaction is usually situated in the shell K (80% probability).
The probability of the occurrence grows with the higher atomic number of the absorbing material (bone, contrast agents etc.).
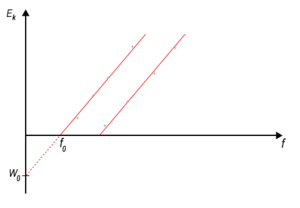
According to the classical physics the kinetic energy of the electromagnetic radiation should be passed on the electrons. Energy of the electromagnetic waves is related to the intensity of the radiation, i. e. energy of the emitted electrons should be a correlative of the intensity of the stimulating radiation. However, experiments showed, that the electron's kinetic energy is related to the frequency and not the intensity of the radiation shining on the material.
For every metal exists a certain minimum of frequency (threshold frequency f0). The photoelectric effect occurs only when light above a the threshold frequency is shone on the metal. The energy of the emitted electrons depends on the frequency of the incident light. If the light frequency f is higher than the threshold frequency f0, the energy of the photoelectrons ranges from zero to certain maximum Emax.
Types of photoelectric effect[edit | edit source]
According to the way of electrons formation by the absorption of the electromagnetic radiation we can distinguish:
a) external photoelectric effect: on the surface of the material, electrons are emitted out of the matter
b) internal photoelectric effect: emission within the material, emitted electrons are left in the material as conductive electrons (semiconductors etc.)
Inverse photoelectric effect[edit | edit source]
Inverse photoelectric effect is the opposite to the photoelectric effect. In this case electrons absorbed by the atom cause the emission of photons.
Explanation of the effect[edit | edit source]
In 1905 Albert Einstein based his thoughts on Planck’s quantum theory and the idea of electromagnetic wave behaving like a complex of particles (light quanta), where each has its own energy and momentum. These particles are unusual, because their velocity is always equal to the velocity of light and there is no way to stop, deccelerate or accelarate them. According to the theory of relativity they must have zero rest mas. These particles were in 1926 called photons. The amount of the energy quantum depends on the frequency (wave length) of electromagnetic radiation:
The energy of light shining on the material is passed on the surface electrons. An electron is only ejected, if it acquires more energy than the work function (the electron binding energy) of the material. This energy is directly propotional to the light frequency and inversely propotional to the light wave length. The minimum frequency, that is necessary to liberate the electron, is called treshold frequency. If the absorbed energy is higher than the minimum amount of energy needed to liberate the electron, the rest contributes to the electron’s kinetic energy as a free particle.
Photoelectric equation: (hf is the energy of absorbed photon, hf0 is the work function- the minimum amount of energy that is necessary to free the electron and Emax is the maximum amount of energy of the ejected electron). It follows, that the energy of the ejected electron depends only on the frequency of the incident light and not its intensity.
Uses[edit | edit source]
Photoelectric effect plays an important part in biophysics. This knowledge can be applied in radiation screenings. X-ray pictures are created on the principle of inverse photoelectric effect, because the surface is bombarded by electrons and so the X-rays arise. Different tissues have different absorbance and that is why we can distinguish different structures on the X-ray pictures. In contrast to Compton effect there are no free electrons left, photon perishes and it never comes to collisions and changes of direction and wave length.
Sources[edit | edit source]
- NAVRÁTIL, Leoš a Jozef ROSINA, et al. Medicínská biofyzika. 1. vydání. Praha : Grada, 2005. 524 s. s. 350-351. ISBN 80-247-1152-4.
- Fotoelektrický jev [online]. [cit. 2018-11-27]. Dostupné z: https://www.wikiskripta.eu/w/Fotoelektrick%C3%BD_jev
- Photoelectriv effect [online]. [cit. 2018-11-27]. Dostupné z: https://physics.info/photoelectric/