Microscopic methods
Microscopy is a collection of applications of optics that are used to visualize structures that are not visible to the naked eye. The eye (biophysics) can distinguish the structure of individual points (details) that are 0.2 mm apart, and in order to be able to distinguish even smaller structures, it is necessary to increase the viewing angle. Different methods of microscopic imaging are distinguished based on the type of radiation arriving at the object (light, ultraviolet radiation (biophysics), polarized light, infrared radiation etc.), or according to the way the optical system is arranged (transmitted light, reflected light, emitted by fluorescence, etc.). Where the resolving power of light microscopy is not sufficient (distinguishes 200 nm), electron microscopy is usedor atomic force microscopy, which will resolve details at the 0.1 nm level.
Optical microscopy[edit | edit source]
Optical microscopy makes it possible to observe things that our eyes can no longer distinguish (distinguishing ability, i.e. the ability to distinguish 2 points lying next to each other, is 0.25 mm in humans). The resolving power of light microscopy is approximately 0,25 µm, which is determined by the wavelength of the radiation that passes through the microscope (in this case light, a stream of photons), but also by the properties of the objective (see below). The resolution of light microscopy is therefore 1000 times greater, than the resolution of the human eye. The maximum useful magnification, that can be achieved in light microscopy is up to 2000 with special microscopes. For a higher level of detail, electron microscopy or atomic force microscopy is used.
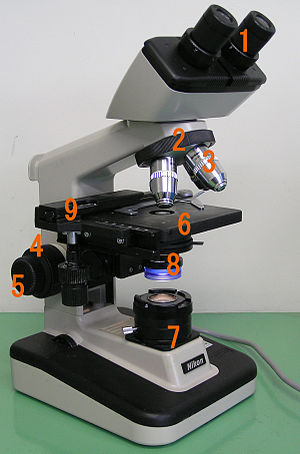
A light microscope consists of three connected optical systems: an illumination system (condenser), an objective and an eyepiece. It also has a mechanical, part that completes the microscope.
The lighting system (under number 7 in the picture) is used to illuminate the specimen, most often against the direction of observation. The preparation that is illuminated in this way must be partially transparent.
The lens (in the picture under the number 3) is a system of lenses with a very short focal length, which together work as a connecting lens and thus display the object (specimen) inverted, real and magnified. The resulting image is projected between the focal point of the eyepiece and the eyepiece.
The eyepieces (pictured under number 1) are also made up of a set of lenses that act as a contact lens. In this case, it performs the function of a magnifying glass, which creates an apparent, direct and enlarged image.
More detailed information about light microscopy can be found here: Light microscopy.
Imaging methods in light microscopy[edit | edit source]
Light field method[edit | edit source]
The transmitted light method
The bright field method is the introductory method of optical microscopy. Light passes through the observed object (in transmitted light) and a system of two converging lenses creates a real, magnified and inverted image that we observe through the eyepiece. In this method, the object has dark outlines and is located in the bright field as opposed to the dark field, where the object is light and located in the dark field.
Dark field method[edit | edit source]
Dark field method
The principle of this method is that the object is illuminated using a condenser so that only peripheral, very oblique light rays enter the plane of the object , until the central ones are absorbed, and therefore are not used at all for imaging. The object is thus illuminated from the sides and the rays are reflected and refracted from it. Thus, the light that is scattered by the object enters the lens. The subject appears to be glowing against a dark background, and is therefore very visible. Therefore, when observing in the dark field, the empty field of view is dark, and only the light that is scattered when it hits the specimen and then partially passes through the lens creates an image.
When observing in a dark field, those parts of the object shine on a dark background, where there is a sufficient difference when the light passes through the observed object, such as its edges.
For dry objects with a numerical aperture of up to 0.65, special condensers are not needed, the output lens of the condenser is shaded by a dark field screen, which is an optional part of the microscope. The source must have sufficient power, as only a fraction of the light intensity of the source is used for this observation. When using high numerical aperture lenses, mainly immersion lenses, immersion cardioid condensers are used for dark field. These condensers have a numerical aperture of 1.05 and their front lens is connected to the underside of the specimen by immersion oil. The numerical aperture of the objective must always be smaller than the aperture of the condenser, otherwise there would be illumination in the bright field.
The method is used for the observation of small objects and their surface structures, e.g., protozoa, bacteria, plant tissues, pollen grains, etc.
Phase contrast method[edit | edit source]
Phase contrast method
This method of microscopy, discovered in 1932 (and awarded the Nobel Prize in 1953) by the Dutch physicist Frits Zernike, allows the clear entry of even the smallest structures, which are almost invisible in ordinary light.
The method is used to highlight the contrast of small phase objects, in which the details do not differ from the surroundings by absorption, but cause a phase change. The method converts differences in the phase shift of light passing through different parts of the object, which we cannot see, into differences in intensities that we can observe.
A more precise principle of operation consists in inserting a special plate with a circular slot on the condenser, on which a bending spectrum is created. Non-colored objects, which differ from their surroundings in refractive index and thickness, cause a change in the phase of the passing light wave, which the human eye does not register. A special plate ensures a shift of the light phase of the direct image of the light source by ¼ wavelength compared to the light phase of the bending images. In the case of wave interference in the image plane, parts of the object that change the phases of light in different ways will show different light intensities. This gives us a contrast image of the phase object.
The advantage of this method is that it does not damage living biological objects and enables their observation over time. This is, for example, important when studying cell and tissue cultures. Phase microscopy also allows us to observe the entire course of mitosis.
Ultraviolet microscopy[edit | edit source]
Ultraviolet microscopy uses UV radiation as a light source, which is characterized by a shorter wavelength than visible light in the range of approximately 400 – 10 nm, which increases the resolution of the microscope. UV radiation is invisible to humans (although some animals can perceive it) and therefore it is necessary to record the resulting image photographically or using a special CCD camera. Since ordinary glass does not transmit UV radiation, it is replaced by quartz glass optics (or other suitable materials). With these exceptions, the basic arrangement (object, objective, eyepiece) is preserved as in optical microscopes.
Infrared microscopy[edit | edit source]
The basic characteristic is the use of infrared radiation pro zobrazení předmětu. to display the object. Infrared radiation, the wavelength of which ranges from 760 nm to 1 mm (IRM mainly uses wavelengths in the range of 760-1100 nm, the so-called near range), is invisible and has significant thermal effects (the human body perceives it as radiant heat). It penetrates some objects more easily than visible light and can therefore also be used to study stronger preparations. The use of IRM is also in the possibility of analyzing complex mixtures, when the components of the mixture have different absorption properties in the infrared region.
The basic arrangement (object, objective, eyepiece) remains the same as for optical microscopes, but for recording, due to the nature of the radiation, it is necessary to use special photomaterials sensitive to infrared radiation. Another difference is the use of spherical mirrors instead of classic lenses, because infrared radiation is absorbed by the glass and does not refract as visible radiation after passing through the glass.
Inverted microscopy[edit | edit source]
Inverted microscopy usually uses visible radiation and solves the need to observe objects that are not squeezed into the confined space between the slide and the cover glass. It enables the observation of objects in thick-walled containers (e.g., Petri dishes), or objects floating freely in liquids in different dishes. The use of visible radiation also results in a limited possibility of magnification.
Structurally, the mentioned advantages are achieved by the fact that the light source is located above the microscope table, the object is placed in a dish on the table, the objective is placed under the table, and the eyepiece part is placed behind a prismatic or planar reflector (mirror) in a direction suitable for comfortable observation.
You can find more about the inverted microscope here: Inverted microscope
Polarization microscopy[edit | edit source]
Polarization microscopy uses the optical activity of the examined specimen (i.e., the ability of the specimen to bend the plane of polarized light). A polarizing microscope is therefore a combination of a polarimeter and a microscope. Structurally, the PM is completely identical to a standard optical microscope, but two polarizing filters, which have crossed polarization planes, are included in the optical path of the light. The rays of the light source first pass through the so-called polarizer (previously a crystal of a birefringent substance, now a plastic polarizing filter) – here polarized light occurs, then they pass through the observed object, a normal lens, then through the second polarizing filter, the so-called analyzer and then the eyepiece. If the observed object, lying between the polarizer and the analyzer, has polarizing properties, after the passage of light through the specimen, the plane of polarization will be twisted and an image in the black field and a color image of the object will be created. This is caused by the fact that the specimen has caused a violation of the condition of the intersection of the planes and appears colored.
You can find more about polarization microscopy here: Polarization microscopy
Interference microscopy[edit | edit source]
Interference microscopy works on the principle of comparing a beam after passing through air and another after passing through a sample. This method is used when examining transparent structures that we do not want to color for some reason. It is also used to visualize various surfaces and membranes.
A double-beam interference microscope consists of an optical microscope and an interferometer. Using the system of interferometer, condenser and Wollaston prism, the beam arriving at the specimen is divided into two. One passes through the sample, which changes its phase. The second passes outside and therefore serves as a reference. This method works on the principle of a dark field – with a suitable setting of the interferometer, the incoming beam is composed of two beams that are shifted in relation to each other by half a wavelength, so if the sample is not placed, the beams will "interfere" and we will not see anything in the field of view. However, when one beam passes through the specimen and its wavelength changes, the total wavelength of the resulting beam also changes, and in the interferometer, therefore, the eyepiece will no longer be "dark" but the image of the examined sample.
There are also multibeam microscopes that compare multiple beams and microscopes using interference phase contrast, which allows certain cell structures to be highlighted.
You can find more about interference microscopy here: Interference microscopy
Fluorescence microscopy[edit | edit source]
Fluorescence is a process when a substance emits radiation with a longer wavelength after absorbing a quantum of radiation. We can talk about primary fluorescence, when the substance in the preparation itself functions as a fluorochrome (e.g., chlorophyll), and secondary when a fluorochrome is added to the sample, which marks originally non-fluorescent structures (e.g., immunoglobulins with a fluorescent marker in immunohistochemistry).
The phenomenon itself is dependent on the electron shell of individual atoms. When an electron in the shell absorbs a photon, its energy increases and the excited electron "jumps" into an orbital with a higher energy. Gradually, however, it loses energy in the form of a photon with a lower energy and a longer wavelength, and the electron falls into its original orbital. Photons with lower energy are emitted in the form of light that we observe in a microscope.
A fluorescence microscope is an optical microscope with a strong source of radiation (mostly mercury or xenon lamps), a monochromator (it transmits only one wavelength – the color of light) and a condenser.
This microscope is used, for example, to detect individual proteins or molecules in a cell.
Laser confocal scanning microscopy[edit | edit source]
Confocal microscopy makes it possible to observe objects with a high resolution and without the disturbing effects of just out-of-focus planes of the specimen.
This is achieved in such a way that the light emitted by the laser towards the specimen first passes through a narrow slit - a point aperture, which concentrates the beam to one point in the sample. From there, the rays pass into the objective, where a second aperture is placed, which eliminates the rays that do not come from that point in the sample. The rays that pass through are detected on a photomultiplier and the position and shape information is sent to a computer.
In this way, enough points are analyzed to cover the entire sample and the computer can create the resulting figure.
You can find more about the confocal microscope here: Confocal microscope
Electron microscopy[edit | edit source]
You can find more about electron microscopy here: Electron microscopy
Thanks to microscopy, we are able to observe things that are beyond the resolution of our eye, and thus are invisible to us with the ordinary eye. Microscopy uses angular magnification methods. In doing so , we increase the angle of view, which is covered by the rays coming from the peripheral points of the object and passing through the optical center of the eye lens. The angular magnification of optical instruments is characterized by the following formula:
γ = τ´/τ
Where τ' is the magnified viewing angle when viewed by an optical device and τ is the viewing angle when viewed by the eye. If we are talking about microscopy, the angular magnification of a microscope is:
γ = Δd /f1 x f2
where f1 is the image focal length of the objective, f2 is the object focal length of the eyepiece, Δ is the optical interval of the microscope, and d is the conventional viewing distance. Depending on the type of radiation hitting the objective, we distinguish different types of microscopy, e.g., light, polarization, fluorescence and electron.
Electron microscopy
Electron microscopy works on a similar principle to light microscopy, which uses the principle of a magnifying glass to increase the angle of view. In the case of a light microscope, the eyepiece acts as a magnifying glass through which the observer looks at the image formed by the objective. Both the eyepiece and the objective represent a system of two connecting lenses. In electron microscopy, it is similar, but unlike light microscopy, which uses a stream of photons and a connecting glass lens, in electron microscopy, the stream of photons is replaced by a stream of electrons, and electromagnetic lenses (magnets) are used to direct the rays through the sample onto the screen. Under the electromagnetic lens, we can basically imagine a coil that creates an appropriately shaped magnetic field that affects the path of electrons. Magnification and resolution are significantly better in an electron microscope, mainly due to the limiting resolution. It is directly proportional to the wavelength of the incident radiation. Since electrons have a shorter wavelength than light, their resolution is many times greater, reaching up to 0.05 nm. Magnification in high-end microscopes can reach up to 10,000,000x.
The two basic types of electron microscopes include the transmission microscope and the scanning microscope. In a transmission electron microscope, the particles pass directly through the sample and only then are they captured. In this type of microscopy, the accelerating voltage must be large enough for the electrons to have sufficient energy to penetrate through the sample, and the sample must also be very thin, 10-500 nm. While the transmission microscope uses the method of transmitted electrons, the scanning method of reflected electrons. In a scanning electron microscope, particles are directed to pass through the sample at a certain angle and are then reflected, creating a 3D image.
Electron microscopy is used in many areas such as materials research or biological applications. It is able to provide comprehensive information about the microstructure, chemical composition and other properties of the examined sample.
Atomic force microscopy (AFM) from the group of probe microscopy methods[edit | edit source]
The essence of the microscope is a silicon nitride probe placed on a spring made of the same material. A laser is aimed at the spring and its beam is reflected into the photodetector. The size of the probe is in the order of individual nanometers.
In non-contact mode, the probe passes over the surface of the examined substance and the spring bends due to capillary and Van der Waals forces (in the order of nanonewtons). The beam from the laser changes the angle of incidence and reflection and hits the photodetector in a different place.
In another, contact mode, the spring flexes in line with the surface as the probe touches deeper and higher places.
The computer will use the information about the places of impact on the detector to model the examined surface. AFM modifications also enable the visualization of magnetic domains or the detection of individual molecules based on the specific behavior of the probe.
The advantage of this method is a magnification of up to a billionth, which allows individual molecules to be displayed, the resulting 3D image and the possibility to measure in different environments (vacuum,
liquid, air...) without special treatment of the sample. The disadvantage is the very high purchase price and low speed.
You can find more about scanning probe microscopy here: Scanning probe microscopy
Links[edit | edit source]
Source[edit | edit source]
- KYMPLOVÁ, Jaroslava. Katalog metod v biofyzice [online]. [cit. 2012-09-20]. <https://portal.lf1.cuni.cz/clanek-793-katalog-metod-v-biofyzice>.
- ROSINA, Leoš. Medicínská biofyzika. 1. edition. Prague : Grada, 2005. Chapter 5.3.1. pp. 241-251. ISBN 978-80-247-1152-2.
- KOČÁREK, Eduard – PÁNEK, Martin – NOVOTNÁ, Drahuše. Klinická cytogenetika I.. 1. edition. Prague : Nakladtelství Karolinum, 2006. Chapter 2. pp. 16-21. ISBN 80-246-1069-8.
https://www.mikroskop-mikroskopy.cz/fazovy-kontrast/
https://www.fch.vut.cz/~zmeskal/obring/prednasky_2004/mikroskopie_2 (martin julinek).pdf
https://www.mikroskop-mikroskopy.cz/temne-pole/
http://biologie.upol.cz/mikroskopie/temne%20pole.htm
http://xarquon.jcu.cz/edu/zbb/fazovyk.pdf
http://fyzika.fce.vutbr.cz/file/kusak/AFM_mikroskopie.pdf
http://web.natur.cuni.cz/~parazit/parpages/mikroskopickatechnika/fluorescencni.htm
https://www.wikiskripta.eu/w/Interferen%C4%8Dn%C3%AD_mikroskopie
https://dml.cz/bitstream/handle/10338.dmlcz/138993/PokrokyMFA_10-1965-2_1.pdf