MEMBRANES & POTENTIALS
The cell is the basic unit of life. All animals are composed of cells. All cells exist in an aqueous (fluid) environment. Intracellular space is separated from the external one by the membrane. Beside the separation, the membrane has another important roles corresponding with the function of the cell.
Specialized functions of the cell are:
- Gas transport (erythrocytes)
- Contraction (muscle cells)
- Energy storage (fat cells)
- Hormone production (endocrine cells)
- Transmission of action potentials (nerve cells) etc.
Water is the major component of a cell (and of an entire organism). More than 99% of the molecules in our body are water molecules. The human body averages about 60% water by weight (about 70 - 74% fat-free weight); average females have slightly lower water content than males. Water content decreases with increasing age.
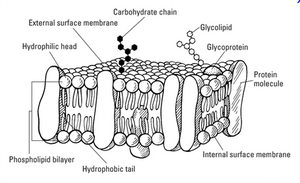
Every cell is surrounded by a membrane: the plasma membrane (Figure 2.1). The plasma membrane can surround the entire cell or the individual organelles within the cell. It is made up of lipid and protein molecules which give the membrane its ability to be selectively permeable. Recent studies suggest, however, that by virtue of their acyl chain composition, membrane bilayers may significantly impact the thermal biology, the metabolic rate, and even the aging process. As the term "Fluid-Mosaic Model" suggests, the plasma membrane is a fluid rather than a rigid structure. In order for membranes to function properly, they must be neither too fluid nor too rigid.
The plasma membrane's roles are:
- Separate the cell from its external environment.
- Regulate the materials that enter or leave the cell.
- Maintain homeostasis.
- Compartmentalize the cell.
- Conduct chemical reactions.
- Participation in cell communication / cell signalling.
- Transfer or storage energy.
The nature of biological membranes is amphiphilic as they form bilayers that contain an internal hydrophobic layer and an external hydrophilic layer. This structure makes transport possible by simple or passive diffusion, which consists of the diffusion of substances through the membrane without expending metabolic energy and without the aid of transport proteins. If the transported substance has a net electrical charge, it will move not only in response to a concentration gradient, but also to an electrochemical gradient due to the membrane potential.
Typical membrane components:
- Phospholipid
- Cholesterol
- Glycolipid
- Sugar
- Polytopic protein (transmembrane protein)
- Monotopic protein (e.g. a glycoprotein)
- Monotopic protein anchored by a phospholipid
- Peripheral monotopic protein (e.g. a glycoprotein)
Phospholipids: The plasma membrane is made up of phospholipids that have amphipathic properties. The membrane is amphipathic because its fatty acid chains create hydrophobic properties and hydrophobic regions in the cell membrane. The phospholipids are arranged in a way where the hydrophobic oily tails of phospholipids face each other while the hydrophilic heads are in contact with the aqueous external environment. This formation gives formation to the lipid bilayer structure of cell membranes that are roughly 10nm thick.
Proteins: Proteins in membranes are embedded into or attached to the membrane. The proteins would constantly move and float across the membrane. The two dimensional fluid and liquid crystalline state of the lipids allows phospholipid molecules to move laterally across the membrane surface. According to the extent of incorporation into the membrane there are three different types of proteins: integral, trans-membrane, and peripheral membrane proteins.
- Integral membrane proteins are bound tightly into the membrane: disrupting the bi layer is the only way to remove them. These proteins are amphipathic meaning they have hydrophilic and hydrophobic regions. Integral proteins can be also called intrinsic proteins.
- Trans-membrane proteins, unlike integral proteins, always extend all the way through the membrane. An example would be the alpha helix or beta pleated sheets (pores).
- Peripheral Proteins, also known as extrinsic proteins, peripheral proteins are bound on the outer surfaces of the membrane bilayer, in the external or internal surface of the membrane. They are located or bounded on exposed regions of integral proteins by covalent bonds. These proteins can be removed easily without disrupting the bilayer structure.
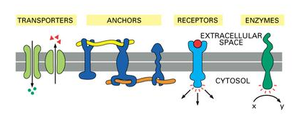
There are different roles of membrane proteins:
- Anchoring - proteins (integrin) anchor the cell to extracellular matrix/micro filaments.
- Passive transport - protein channels for selective passage of material.
- Active transport - proteins pump solutes across membrane with ATP.
- Enzymatic activity - catalyse reactions along or within surface of membrane.
- Signal transduction - receptors bind molecules (hormones) to transmit information.
- Cell recognition - glycoproteins or other proteins that act as an identification tag.
- Intercellular junction - adhesion of proteins that attach cells.
TRANSPORT ACROSS BIOLOGICAL MEMBRANES[edit | edit source]
Transport proteins (Figure 2.2) are proteins that facilitate ions, amino acids, sugars and other polar molecules through the membrane. There are two basic types of transport proteins: carrier and channel proteins.
- Carrier proteins (transporters) undergo conformation in order to allow molecules to go through the membrane. ABC transporters make up carrier proteins and are responsible for the use of energy ATP to transport ions, sugars, and polypeptides across the membrane.
- Channel proteins are gated proteins that open and close to allow materials to enter the cell. The gates are opened if triggered by electrical signals, chemical stimuli or mechanical stimuli.
In cellular biology the term membrane transport refers to the collection of mechanisms that regulate the passage of solutes such as ions and small molecules through biological membranes. The regulation of passage through the membrane is due to selective membrane permeability - a characteristic of biological membranes which allows them to separate substances of distinct chemical nature. In other words, they can be permeable to certain substances but not to others.
The movements of most solutes through the membrane are mediated by membrane transport proteins which are specialized to varying degrees in the transport of specific molecules. As the diversity and physiology of the distinct cells is highly related to their capacities to attract different external elements, it is postulated that there is a group of specific transport proteins for each cell type and for every specific physiological stage. This differential expression is regulated through the differential transcription of the genes coding for these proteins and its translation, for instance, through genetic-molecular mechanisms, but also at the cell biology level: the production of these proteins can be activated by cellular signalling pathways, at the biochemical level, or even by being situated in cytoplasmic vesicles.
Thermodynamically the flow of substances from one compartment to another can occur in the direction of a concentration or electrochemical gradient or against it. If the exchange of substances occurs in the direction of the gradient, that is, in the direction of decreasing potential, there is no requirement for an input of energy from outside the system; if, however, the transport is against the gradient, it will require the input of energy, metabolic energy in this case. For example, a classic chemical mechanism for separation that does not require the addition of external energy is dialysis. In this system a semipermeable membrane separates two solutions of different concentration of the same solute. If the membrane allows the passage of water but not the solute the water will move into the compartment with the greatest solute concentration in order to establish an equilibrium in which the energy of the system is at a minimum. This takes place because the water moves from a high solvent concentration to a low one (in terms of the solute, the opposite occurs) and because the water is moving along a gradient there is no need for an external input of energy.
As few molecules are able to diffuse through a lipid membrane the majority of the transport processes involve transport proteins. These trans-membrane proteins possess a large number of alpha helices immersed in the lipid matrix. These proteins can be involved in transport in a number of ways: they act as pumps driven by ATP, that is, by metabolic energy, or as channels of facilitated diffusion.
PASSIVE DIFFUSION[edit | edit source]
A semipermeable membrane separates two compartments of different solute concentrations: over time, the solute will diffuse until equilibrium is reached.
Passive diffusion is a spontaneous phenomenon that increases the entropy of a system and decreases the free energy. The transport process is influenced by the characteristics of the transport substance and the nature of the bilayer. Membrane proteins are not involved in passive diffusion.
The diffusion velocity of a pure phospholipid membrane will depend on:
- Concentration gradient.
- Hydrophobicity.
- Size of the molecule.
- Charge (if the molecule has a net charge).
ACTIVE TRANSPORT AND CO-TRANSPORT[edit | edit source]
In active transport a solute is moved against a concentration or electrochemical gradient. In doing so the transport proteins involved consume metabolic energy, usually ATP. In primary active transport the hydrolysis of the energy provider (e.g. ATP) takes place directly in order to transport the solute in question. For instance, when the transport proteins are ATPase enzymes. Where the hydrolysis of the energy provider is indirect as is the case in secondary active transport, use is made of the energy stored in an electrochemical gradient. For example, in co-transport use is made of the gradients of certain solutes to transport a target compound against its gradient, causing the dissipation of the solute gradient. It may appear that, in this example, there is no energy use, but hydrolysis of the energy provider is required to establish the gradient of the solute transported along with the target compound. The gradient of the co-transported solute will be generated through the use of certain types of proteins called biochemical pumps.
The discovery of the existence of this type of transporter protein came from the study of the kinetics of cross-membrane molecule transport. For certain solutes it was noted that the transport velocity reached a plateau at a particular concentration above which there was no significant increase in uptake rate, indicating a log curve type response. This was interpreted as showing that the transport was mediated by the formation of a substrate-transporter complex, which is conceptually the same as the enzyme-substrate complex of enzyme kinetics. Therefore, each transport protein has an affinity constant for a solute that is equal to the concentration of the solute when the transport velocity is half its maximum value.
Transporter proteins - uniport, symport and antiport of molecules through membranes: A transport protein can move various ions and molecules; they are distinguished according to their directionality.
- Uniporters are carrier proteins that work by binding to one molecule of solute at a time and transporting it with the solute gradient. Uniporter channels open in response to a stimulus and allow the free flow of specific molecules. Uniporters may not utilize energy other than the solute gradient. Thus they may only transport molecules with the solute gradient, and not against it. There are several ways in which the opening of uniporter channels may be regulated. Voltage regulated channels are regulated by the difference in voltage across the membrane. Stress regulated channels are regulated by physical pressure on the transporter (as in the cochlea of the ear). Ligand regulated channels are regulated by the binding of a ligand to either the intracellular or extracellular side of the cell. Uniporters are involved in many biological processes, including impulse transmission in neurons. Voltage-gated sodium channels are involved in the propagation of a nerve impulse across the neuron. During transmission of the signal from one neuron to the next, calcium is transported into the presynaptic neuron by voltage-gated calcium channels. Potassium leak channels are also regulated by voltage and they help to restore the resting membrane potential after impulse transmission.
- Antiporters (also called exchanger or counter-transporter) are transport proteins which transport a molecule against its gradient and at the same time displaces one or more ions along its gradient, both gradients being opposite,
- Symporters are transport proteins which move a molecule against its gradient while displacing one or more different ions along their gradient which is in the same direction as that of the transported molecule.
Antiporters and symporters can be referred to as co-transporters.
Pumps: A pump is a protein that hydrolyses ATP in order to transport a particular solute through a membrane in order to generate an electrochemical gradient to confer certain membrane potential characteristics on it. This gradient is of interest as an indicator of the state of the cell through parameters such as the Nernst potential (see below). In terms of membrane transport the gradient is of interest as it contributes to increased system entropy in the co-transport of substances against their gradient.
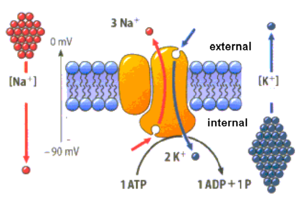
One of the most important pumps in animal cells is the sodium potassium pump (Na/K ATPase) that operates through the following mechanism (Figure 2.3):
- Binding of three Na+ ions to their active sites on the pump which are bound to ATP.
- ATP is hydrolysed leading to phosphorylation of the cytoplasmic side of the pump which induces a structure change in the protein. The phosphorylation is caused by the transfer of the terminal group of ATP to a residue of aspartate in the transport protein and the subsequent release of ADP.
- The structure change in the pump exposes the Na+ to the exterior. The phosphorylated form of the pump has a low affinity for Na+ ions so they are released.
- The pump binds two molecules of K+ to their respective bonding sites on the extracellular face of the transport protein.
- Reverting pump to its previous conformational state, transporting the K+ ions into the cell.
- The unphosphorylated form of the pump has a higher affinity for Na+ ions than K+ ions, so the two bound K+ ions are released into the cytosol.
- ATP binds, and the process starts again.
MEMBRANE POTENTIAL[edit | edit source]
Ions are charged atoms or molecules. Cations carry one or more positive charges. Anions carry one or more negative charges. Like charges repel and unlike charges attract. An ion can thus exert a force on another ion. If ions are separated (i.e. they are some distance apart), they have the potential for doing work. Voltage is a measure of the potential of separated charges for doing work. Every living cell has a membrane (resting) potential. These result from the diffusion of ions across the membrane and they are negative by convention (the inside is negative relative to the outside).
Resting potentials typically range from -50 to -100 mV (millivolts). The membrane potential ultimately depends on the distribution of ions between the intracellular and extracellular environment.
These distributions depend on:
- Differential permeability of the membrane to different ions.
- Presence of non-diffusible ions.
- Action of the Na/K ATPase pump.
- Electrochemical properties of the membrane itself.
An equilibrium potential will be established if we have a membrane which is permeable to potassium but not to sodium (potassium is high inside a living cell and sodium is high outside). A typical resting potential of -70 mV, the electrical and chemical forces acting on potassium are in opposite directions, whereas those for sodium are in the same direction. The resting potential is influenced by the presence of non-diffusible ions (i.e. by proteins, which tend to have a net negative charge, and which, because of their large size, are not able to diffuse outwards). This contributes to the inside negativity of the membrane. The magnitude of this effect can be predicted from the Donnan (or Gibbs-Donnan) Equilibrium (Figure 2.4). The sodium/potassium pump acts to maintain the resting potential by continuously pumping out sodium that enters the cell and replacing potassium that leaks out. However, the pump also directly contributes to the charge separation because it transports the two ions unequally (i.e. it pumps three sodium ions out for every two potassium ions that it brings in). It thus has a direct electrogenic effect.
The plasma membrane, by virtue of its chemical composition and structure, helps to maintain an electrical potential. The membrane has a high electrical resistance and also a large capacitance (i.e. ability to hold a potential). This latter property is related to the thinness of the membrane (capacitance increases as membrane thickness decreases).
The Nernst equation (2.1) allows us to predict the magnitude of an equilibrium diffusion potential from a particular distribution of ions. The trans-membrane potential can be expressed as
EX = (RT/zF)·ln([X]outside/[X]inside) (2.1)
R = the gas constant, T = absolute temperature, z = valence, F = Faraday's number, and [X] = ionic concentration Fortunately, we can simplify this by considering only univalent ions, by holding temperature constant (e.g. at 25 C), and by combining the gas constant and Faraday's number:
for a cation E = -59 log ([Xi]/[Xo]) (2.2)
for an anion E = +59 log ([Xi]/[Xo]) (2.3)
Typical concentrations for the three major ions are:
For sodium: E = -59 log (15/150) = -59 log (0.1) = +59 mV (2.4)
For potassium: E = -59 log (150/5) = -59 log (30) = -87 mV (2.5)
For chloride: E = +59 log (4/110) = +59 log (0.036) = -85 mV (2.6)
The measured potential for a living cell (-70 mV) is very different from the predicted equilibrium potential for sodium (2.4). We can thus conclude that the diffusion of sodium contributes little to the resting potential. In contrast, the predicted potentials for both potassium (2.5) and chloride (2.6) are reasonably close to -70 mV.
The resting potential of a cell is thus primarily potassium diffusion potential. The undisturbed cell is much more permeable to potassium than to sodium. The ratio is on the order of 30:1. Cells contain both gated and non-gated (aka open or "leak") channels for both ions, but far more non-gated channels for potassium than for sodium. Summarizing for resting membrane potential, the Na+/K+ ATPase pump establishes an unequal distribution of ions and the basis for a resting potential, the electrogenic effect of the pump contributes to the potential. At a steady state, there is a balance between ion pumping and ion fluxes through membrane channels.
ACTION POTENTIAL[edit | edit source]
An action potential (AP) is a short-lasting event in which the electrical membrane potential of a cell rapidly rises and falls, following a consistent trajectory. APs occur in several types of cells, called excitable cells, which include neurons, muscle cells, and endocrine cells. In neurons, they play a central role in cell-to-cell communication. In other types of cells, their main function is to activate intracellular processes. In muscle cells, for example, an action potential is the first step in the chain of events leading to contraction.
APs in neurons are also known as "nerve impulses" or "spikes", and the temporal sequence of action potentials generated by a neuron is called its "spike train". A neuron that emits an action potential is often said to "fire". APs are generated by special types of voltage-gated ion channels embedded in a cell's plasma membrane. These channels are shut when the membrane potential is near the resting potential of the cell, but they rapidly begin to open if the membrane potential increases to a precisely defined threshold value. When the channels open, they allow an inward flow of sodium ions, which changes the electrochemical gradient, which in turn produces a further rise in the membrane potential. This then causes more channels to open, producing a greater electric current, and so on. The process proceeds explosively until all of the available ion channels are open, resulting in a large upswing in the membrane potential. The rapid influx of sodium ions causes the polarity of the plasma membrane to reverse, and the ion channels then rapidly inactivate. As the sodium channels close, sodium ions can no longer enter the neuron, and they are actively transported out of the plasma membrane. Potassium channels are then activated, and there is an outward current of potassium ions, returning the electrochemical gradient to the resting state. After an AP has occurred, there is a transient negative shift, called the after hyperpolarization or refractory period, due to additional potassium currents. This is the mechanism that prevents an action potential from traveling back the way it just came.
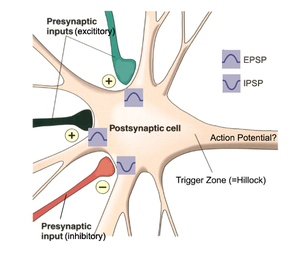
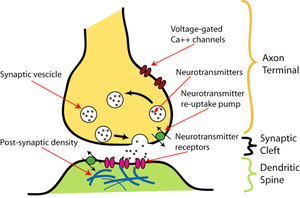
APs in neuron are most commonly initiated by excitatory postsynaptic potentials from a presynaptic neuron, typically by release of neurotransmitter molecules which are consequently bind to receptors on the postsynaptic cell. This binding opens various types of ion channels. This opening has the further effect of changing the local permeability of the cell membrane and, thus, the membrane potential. If the binding increases the voltage (depolarizes the membrane), the synapse is excitatory. If the binding decreases the voltage (hyperpolarizes the membrane), then it is inhibitory. Whether the voltage is increased or decreased, the change propagates passively to nearby regions of the membrane. There are two possibilities to elicit AP. First, some fraction of an excitatory voltage may reach the axon hillock and may (in rare cases) depolarize the membrane enough to provoke a new AP. Secondly, more typically, the excitatory potentials from several synapses work together at nearly the same time to provoke a new AP (time coincidence, see Figure 2.5).
“All-or-none" principle means the amplitude of an AP is independent of the amount of current that produced it. The frequency of APs is correlated with the intensity of a stimulus.
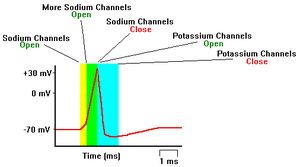
Generation of Action Potential: As the membrane potential of the cell is increased, sodium ion channels open, allowing the entry of sodium ions into the cell. This is followed by the opening of potassium ion channels that permit the exit of potassium ions from the cell (see Figure 2.6).
The inward flow of sodium ions increases the concentration of positively charged cations in the cell and causes depolarization, where the potential of the cell is higher than the cell's resting potential. The sodium channels close at the peak of the action potential, while potassium continues to leave the cell. The efflux of potassium ions decreases the membrane potential or hyperpolarizes the cell. For small voltage increases from rest, the potassium current exceeds the sodium current and the voltage returns to its normal resting value, typically −70 mV. However, if the voltage increases past a critical threshold, typically 15 mV higher than the resting value, the sodium current dominates. This results in a runaway condition whereby the positive feedback from the sodium current activates even more sodium channels.
Refractory period: During a short period after the occurrence of a spike the cell cannot be stimulated. This is the refractory period (1-5ms) consisting of an absolute and relative phase. In the absolute phase, the Na+ channels cannot be opened by a stimulus irrespective of applied voltage. In the subsequent relative phase, spikes can be initiated, since Na+ channels are reactivated (in a stochastic manner) but the threshold is greater. This is caused by the slightly hyperpolarized state due to still higher than resting value for potassium, so more voltage is required to reach threshold, and also the threshold itself is higher than usual because some of the Na+ channels will still be inactivated.
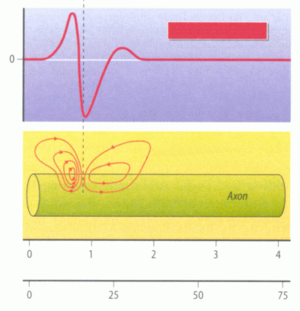
Propagation of Action Potential: Propagation of the nerve (or muscle) membrane potential disturbance is strongly reduced along distances, it is slow and (due to temporal filtering) and the peak time for example at the nerve terminal will poorly defined. With spikes (APs) information transport is faster and with higher temporal resolution and as a consequence more information can be transmitted. Spikes arise from the somatic potential, the sum of the dendritic potentials, at the axon hillock between the axon and soma. Then they propagate along axons (sometimes certain types of dendrites), muscle fibres and also heart muscle fibres. Figure 2.7 visualizes this propagation.
Neuron: Neuron is an electrically excitable cell that processes and transmits information through electrical and chemical signals. A chemical signal occurs via a synapse, a specialized connection with other cells (Figure 2.8). A typical neuron possesses a cell body (soma), dendrites, and an axon. Dendrites are thin structures that arise from the cell body, often extending for hundreds of micrometres and branching multiple times, giving rise to a complex "dendritic tree". An axon is a special cellular extension that arises from the cell body at a site called the axon hillock and travels for a distance, as far as 1 meter in humans or even more in other species.
The cell body of a neuron frequently gives rise to multiple dendrites, but never to more than one axon, although the axon may branch hundreds of times before it terminates. At the majority of synapses, signals are sent from the axon of one neuron to a dendrite of another. All neurons are electrically excitable, maintaining voltage gradients across their membranes by means of metabolically driven ion pumps. Changes in the cross-membrane voltage can alter the function of voltage-dependent ion channels and consequently to generate AP which travels rapidly along the cell's axon, and activates synaptic connections with other cells when it arrives.
The main functions of neuron are:
- Thresholding of input signals.
- Integration (temporal and spatial) of input signals.
- The generation of action potentials.
Neurons can be classified according to their electrophysiological characteristics, for example as tonic or regular spiking, phasic or bursting or fast spiking.