Interaction of Ionizing Radiation
During the passage of ionizing radiation through matter, there is an interaction between the particles or photons of the radiation and the structures of the surrounding atoms , i.e. the nucleus and the electron shell. The course of the interaction itself depends on the nature of the radiation , its kinetic energy and the composition of the substance in which the interaction takes place.
The interaction is evaluated from two perspectives:
- from the point of view of radiation – changes in energy, number of particles and direction of passing radiation;
- from the point of view of the environment – movements of subatomic particles and subsequent reactions.
According to the interaction , we divide ionizing radiation into:
- directly ionizing – electrically charged particles – radiation α , β - and β + , protons, nuclear fragments;
- indirectly ionizing - electroneutral radiation - X- ray , γ radiation , neutron radiation.
According to the place of interaction , we divide into:
- interacting with the core ;
- interacting with the atomic shell .
Overall, ionizing radiation can therefore be divided into three groups:
- electromagnetic (photon) radiation – X- rays and γ radiation;
- charged particles – p, α, β;
- uncharged particles - neutrons.
Interaction of Electromagnetic Radiation[edit | edit source]
The interaction occurs in the nucleus and its electromagnetic field or in the shell of the atom. The interactions of both types of radiation (X-ray and γ) are very similar, they differ in the place of origin (X-ray from the envelope, γ from the core) and frequency.
In total, we distinguish six types of interactions of photon radiation with matter (see table). Only the three most important ones will be discussed in more detail: the photoelectric effect, Compton scattering and the formation of electron-positron pairs.
| absorption | Flexion Collision | Inelastic collision | |
|---|---|---|---|
| Electronic envelope | Photoelctric phenomenon | Rayleigh Scattering | Compton Scattering |
| Atomic Nucleus | Photoelectric Interaction | Nuclear Resonance Scattering | |
| EMG Field | Formation of Electron-Positron pairs |
Photoelectric Phenomenon[edit | edit source]
Introduction[edit | edit source]
The photoelectric phenomenon (photoeffect) is one of three possible interactions of γ radiation with the electron shell of an atom . Of these three interactions, the photon usually has the weakest energy. It is a physical phenomenon in which electrons are released (radiated, emitted) from a substance (most often a metal ) as a result of absorption of electromagnetic radiation by the substance. Electrons emitted from the nuclear shell are then referred to as photoelectrons . Their release is referred to as photoelectric emission (photoemission) .
History[edit | edit source]
Heinrich Hertz is considered to be the discoverer of the photoelectric phenomenon , who, during his experiments (in 1887), whose goal was to experimentally prove the existence of electromagnetic waves predicted by Maxwell , noticed that the irradiation of a spark gap with ultraviolet radiation facilitates the jump of the spark - i.e. the transfer of electric charge between the electrodes.
In 1899, Joseph John Thomson took a decisive step towards clarifying the nature of the phenomenon. Thomson experimentally identified electrons in negative charge carriers escaping from an irradiated metal sample.
The actual nature of the photoelectric phenomenon was described in 1905 by Albert Einstein (he won the Nobel Prize for this discovery in 1921).
Description Of the Phenomenon[edit | edit source]
The photoelectric phenomenon occurs when the entire energy of a quantum of γ radiation is transferred to an electron from the electron shell of an absorbing material or possibly to a free electron (e.g. in metals). Part of the energy is used to release the electron (by performing the so-called output work W v ) and part is transformed into the kinetic energy E of the resulting photoelectron . The photon of γ radiation thus disappears and its energy is taken over by a photoelectron, which ionizes its surroundings.
Einstein's equation for the photoeffect expresses the law of conservation of energy .
(h is Planck's constant)
An atom from which an electron has been knocked out is in an excited state and transitions to the ground state by emitting electromagnetic radiation with a frequency corresponding to the energy difference between the excited and ground states.
(The free space after the electron is filled by another electron that jumped here from another shell of the atomic shell. During this jump, energy is emitted in the form of characteristic radiation. Instead of characteristic radiation, an alternative phenomenon can occur - the energy is transferred to an electron on a higher shell, which then it releases and emits as a so-called Auger electron.)
A photon interacts with an electron on the K, L and M shells, that is, with electrons that lie close to the nucleus of an atom. It most often occurs on the K peel (80% probability).
Phopho effect is more likely in materials with a higher proton number of the absorption material (bone, contrast agents).
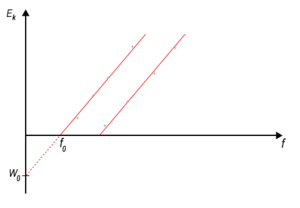
According to the ideas of classical physics , the kinetic energy of the incident electromagnetic wave should be transferred to the electrons . The energy of electromagnetic waves is related to the intensity of radiation , i.e. the energy of the emitted electrons should depend on the intensity of the incident radiation. However, experiments have shown that the kinetic energy of the emitted electrons is dependent on the frequency and not on the intensity of the incident radiation.
For each metal there is a certain cut-off frequency f 0 such that electrons are released only at the frequency f 0 and higher frequencies. The energy of the emitted electrons also depends on the frequency of the electromagnetic radiation used. If the frequency f of the incident radiation is higher than the limiting frequency f 0 , the photoelectrons have an energy ranging from zero to a certain maximum value Emax.
The dependence of the observed phenomenon on the radiation frequency could not be explained classically.
Types of Photo effect[edit | edit source]
According to the way electrons are created due to incident electromagnetic radiation, we can distinguish:
- 1. external photoelectric phenomenon - the phenomenon takes place on the surface of the substance, electrons are released into the surroundings
- 2. internal photoelectric phenomenon - the released electrons remain in it as conduction electrons (e.g. semiconductors, in which electrons are released in this way mainly from the PN transition)
Inverse Photoelectric Effect[edit | edit source]
The inverse (reversed) photoelectric effect is a phenomenon where electrons strike a substance, causing photons to be emitted .
Explanation Of The Phenomenon[edit | edit source]
In 1905 , Albert Einstein started from Planck's quantum hypothesis and from the idea that an electromagnetic wave of frequency f and wavelength λ behaves like a set of particles (light quanta) , each of which has its own energy and momentum. These particles have special properties, above all they are still moving at the speed of light and cannot be stopped, slowed down or accelerated in any way. According to the theory of relativity, it must have zero rest mass. These particles were named photons in 1926 . The size of a quantum of energy depends on the frequency (wavelength) of electromagnetic radiation, while:
When light hits the surface of a substance , it transfers energy to the surface electrons of the substance under investigation. So-called ionization energy is needed to release an electron from a bond in an atom . This necessary energy to release an electron can be created if the wavelength of the light is small enough . In that case, the frequency and energy can reach a sufficiently high value. By transferring such energy to the electrons, it is possible to overcome the so-called photoelectric barrier to perform output work . The minimum frequency at which the incident photons impart output energy to the electrons is referred to as the threshold frequency. If the energy imparted to the electron is greater than the energy needed to release it, then some of the photoelectron's energy remains as kinetic energy .
The equation of the photoelectric effect: (hf is the energy of the incident photon, hf 0 is the output work - the minimum energy required to release an electron, E max is the maximum possible energy of the released electron) It follows from this equation that the energy of the released electron depends only on the frequency of the incident radiation , and not on the intensity of this radiation.
Uses[edit | edit source]
The photoelectric phenomenon plays an important role in the field of biophysics. An example is the application of these phenomena during radiation examinations of a patient. X-ray images are created on the principle of an inverted photoelectric effect, when the surface is bombarded with electrons and X-rays are released. Different tissues have different absorption, which is why we can distinguish structures in the images. The electron completely absorbs the photon and the X-ray photon disappears. Absorption of the photoelectric effect is desirable, unlike Compton scattering , which also takes place. In the Compton phenomenon, free electrons remain and the photon does not disappear, so objects collide and their direction and wavelength change.
Compton scattering[edit | edit source]
Compton scattering describes the collision of a photon with, for example, an electron due to subsequent changes in the wavelength of the resulting photon.
History[edit | edit source]
In 1905, Albert Einstein introduced the idea of the corpuscular wave character of particles to explain the photoelectric effect . Since, according to her, it was possible to consider a photon to be both a wave and a particle, there should be interactions between it and, for example, an electron, which would correspond in nature to elastic collisions, during which total momentum and energy are conserved within the isolated system .
However, according to the ideas of classical physics, after the collision of a photon with an electron, the electron should be oscillated by the frequency of the incident photon and then send out photons again with the same frequency.
In 1922, Arthur Holly Compton decided to test this theory . He created an X-ray scattering experiment on free electrons. It was necessary to use the impact of radiation on materials with very weakly bound electrons. X-rays (λ = 0.07 nm) hit the carbon target. Compton was able to detect duplicate spectral lines : one corresponded to the original wavelength (scattering on tightly bound electrons), the other had a higher wavelength (scattering on free electrons). The correctness of Einstein's theory was thus experimentally confirmed, and Compton won the Nobel Prize in Physics in 1927 .
Compton Shift[edit | edit source]
The existence of a second wavelength was expressed by the equation for the Compton shift:
λ ... the wavelength of the photon before the collision
λ´ … the wavelength of the photon after the collision
φ … scattering angle
h/m 0 c ... Compton wavelength (for an electron = 2.4262 · 10-12 m)
Additions to the Theory[edit | edit source]
In theory, the Compton effect occurs every time a photon collides with an electron, but if the mass of the photon is very small compared to the mass of the electron, this shift is minimal. Because of this, the Compton effect can only be observed using radiation with a high photon mass, such as X-rays or gamma rays .
The secondary photon deviates in the interval 0–180° and its energy depends on the deviation. If there is backscattering (i.e. 180° angle), the photon has the least energy. The secondary photon may be able to repeat the phenomenon again if it has sufficient energy, or it decays through the photoelectric effect .
Use[edit | edit source]
The Compton effect is used in many scientific fields. Examples include radiotherapy (targeted DNA damage of e.g. cancer cells), spectroscopy (detection of ionizing radiation) and astronomy (Compton's gamma observatory).
Electron-positron pairs[edit | edit source]
The formation of electron-positron pairs occurs when high-energy γ radiation interacts with the electron shell of an atom . It is the highest energy possibility of the three γ-ray interactions with the shell.
At photon energies theoretically above 1.02 MeV, but practically much higher, the photon is converted near the atomic nucleus into a positron and an electron . At the same time, it is necessary that this happens near the atomic nucleus or another particle that can take over part of the momentum of the photon (since the momentum of the positron and electron is lower). Spontaneous transformation of a photon into an electron and a positron is not possible when it moves in a vacuum due to the law of conservation of momentum(the sum of the momentums of the resulting electron and positron is less than the momentum supplied by the photon). The transformation itself takes place as a result of the electric field of the atomic nucleus (the greater the charge of the nucleus, the greater the probability of transformation). The kinetic energy of the created electron-positron pair is distributed randomly between the two particles.
The following equation can be used to express the energy balance of the given event:
It follows from the given relationship that the energy of the photon must be greater than the energy that represents the sum of the two rest masses of the electron (the sum of the rest energies of the electron and the positron are still the same).
The resulting particles lose their energy during interactions with the surrounding environment, i.e. ionization or excitation . However, the positron usually combines with the electron during the annihilation process and thus emits two quanta of electromagnetic radiation with an energy of 511 keV. These quanta move in the opposite direction.
Interaction of charged particles[edit | edit source]
Heavier charged particles interact with matter by inelastic collisions. In this way, they transfer their kinetic energy to the surroundings. We call this event collisional energy losses . The charge does not change.
The interaction can also take place in the form of so-called radiation loss , when only the electromagnetic fields of the particles interact. This often happens with light particles, electrons.
Radiation particles do not have to transfer all their energy at once. The energy manifests itself in the target structure as an excitation of either the nucleus or the electrons in the shell. Energy is always lost in the form of heat. If the transferred energy is large enough, an electron can be detached, which then behaves as a β - particle, its kinetic energy is equal to the energy transferred by the impact. This so-called secondary electron radiation is sometimes referred to as δ radiation .
Heavier particles carrying a larger charge interact more often, transfer their energy to the surroundings over short distances and then disappear.
Interaction of Uncharged Particles[edit | edit source]
Neutrons , as the most important representatives of the group of uncharged particles, interact with the surrounding matter only on the basis of strong and weak nuclear forces.
The interaction can take place in the form of elastic and inelastic scattering , emission of a charged particle , radiation (neutron) capture , or nuclear fission .
More detailed information can be found on the LET page .
Flexible dispersion[edit | edit source]
The most likely type of interaction is elastic scattering . It occurs in very small nuclei that are close to a neutron in size, such as hydrogen. The energy transferred by the neutron is completely transformed into the kinetic energy of the struck particle. The atom does not get excited. The reflected neutron continues on with the rest of the energy. This process is called neutron rate moderation . The process continues until the neutron slows down enough to be absorbed by the nucleus. Moderation is used in 235 uranium nuclear reactors , when hydrogen atoms in water molecules slow down fast neutrons produced by fission.
See the Nuclear Reactor page for more detailed information .
Inelastic scattering[edit | edit source]
Inelastic scattering occurs at the cores of heavy elements. Similar to elastic scattering, the neutron transfers part of its kinetic energy and continues on as slowed down. However, the affected nucleus is excited, part of the transferred energy is emitted in the form of a γ photon, the rest is transformed into the kinetic energy of the nucleus.
Charged particle emission[edit | edit source]
A neutron has so much energy that when it hits the nucleus, one or several nuclear elements are knocked out. The kinetic energy of the neutron is therefore used to knock out a proton, an α particle or a deuteron (a deuterium nucleus, one proton and one neutron), the rest of the transferred energy turns into the kinetic energy of the knocked out particle. This can lead to the formation of an unstable nuclide and its further decay.
Radiation Capture[edit | edit source]
The neutron is captured by the nucleus, its kinetic energy is emitted in the form of a γ photon.
Nuclear Fission[edit | edit source]
At an appropriate neutron velocity, relative to the target atomic nucleus, the nucleus can split to produce fission products , which are mostly radioactive isotopes. During fission, so much energy is released from the nucleus that the resulting neutrons have a higher energy than the one that caused the fission. Usually a photon of γ radiation is emitted. If more than one fissionable neutron is released, a so-called avalanche effect occurs with an exponential increase in interactions. This fission chain reaction is used in nuclear weapons. In a moderate form (= not all generated neutrons split other nuclei) it is the basis of a nuclear reactor.
Links[edit | edit source]
Related Articles[edit | edit source]
References[edit | edit source]
- PODZIMEK, František. Biofyzika ionizujícího záření. 1. edition. Vojenská lékařská akademie Jana Evangelisty Purkyně, 1990. 119 pp. ISBN 80-85109-24-7.
- BENEŠ, Jiří – STRÁNSKÝ, Pravoslav – VÍTEK, František. Základy lékařské biofyziky. 2. edition. Karolinum, 2007. 201 pp. ISBN 978-80-246-1386-4.
- NAVRÁTIL, Leoš – ROSINA, Jozef. Medicínská biofyzika. 1. edition. Grada, 2005. 524 pp. ISBN 80-247-1152-4.
Category : Biophysics
Exam Questions from Biophysics
Radiodiagnosis