Hereditary coagulopathy
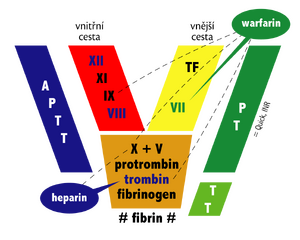
Blood clotting is a process in which the gradual activation of coagulation factors produces thrombin , which converts fibrinogen to fibrin. When the balance of pro- and anti-coagulation factors is disturbed, bleeding or excessive blood clotting may occur.
- Congenital bleeding conditions
- Hemophilia A and B; Von Willebrand disease.
- Congenital thrombophilic conditions
- Leiden mutation ; Prothrombin mutation; Antithrombin deficiency; Protein C deficiency; Protein S deficiency; Hyperhomocysteinemia; Lipoprotein (a) .
Coagulation in children[edit | edit source]
Children have a greater tendency to bleed. The newborn has low levels of factors II, VII, IX, X and contact factors. A healthy child reaches adult values at 3-6 months of age. Compared to adults, newborns have an increased level of von Willebrand factor and a reduced level of antithrombin, protein C and protein S, and a significantly reduced level of plasminogen.[1]
Examination of coagulation and normal values in children[edit | edit source]
- Blood count;
- activated partial thromboplastin time (APTT): 28–42 s; ratio: 1–1.5 (1st day of life)...0.8–1.2 (from 1 month);
- Quick prothrombin time (PT): 11–17 s; INR: 1-1.5 (1st day of life)...0.8-1.2 (from 6 months);
- thrombin time (TT): 10–21 s;
- fibrinogen: 1.5-3.5 g/l (0-1 year)...1.54-4.5 g/l (11-16 years)...1.8-3.5 g/l (18 and over);
- antithrombin: 40-90% (neonate)...80-140% (infant-preschooler)...80-120% (18 years and older);
- D-dimers : < 500 ng/ml;
- proof of activation of the coagulation system (protamine sulfate test, ethanol gelation test).[1]
Congenital bleeding conditions[edit | edit source]
Severe bleeding on the basis of congenital coagulopathy is most often caused by hemophilia in children. There are most patients with von Willebrand's disease in the population, but a large part of them do not have significant bleeding manifestations. Other congenital coagulopathies are rare.
Hemophilia A and B[edit | edit source]
- Congenital deficiency of FVIII (A) and FIX (B); variously severe deficit (mild–severe);
- inheritance gonosomally recessive, X-linked; most often a newly arisen mutation;
- frequency: 1/5000 boys;
- hemophilia A is 5x more common than hemophilia B; the same clinical picture;
- pathophysiology: FVIII/FIX deficiency leads to impaired coagulase formation (impaired activation of FX, which is key in the conversion of fibrinogen to fibrin);
- clinical picture: severe bleeding according to the degree of deficiency (bleeding in case of serious injury → spontaneous bleeding in joints, muscles, bleeding even in case of minimal injury – intracranial bleeding in newborns, extensive cephalhematoma , bleeding from the navel); bleeding into the joints → synovial hypertrophy, destruction of joint cartilage, pain, limitation of mobility ("hemophilic arthropathy"); there is no excessive bleeding from small cuts and abrasions ( primary hemostasis is normal);
- laboratory examination: prolonged APTT; other parameters in the norm; reduced level of FVIII or FIX; DNA analysis; examination of the level of vWF and the level of FVIII and FIX inhibitors;
- therapy: substitution of the missing factor with concentrates; in mild form of hemophilia A – desmopressin acetate; dispensary in hematology centers;
- half-life of FVIII is 8–12 hours; the half-life of FIX is 20–24 hours; frozen plasma is low in FVIII and FIX;
- complications: development of an inhibitor against FVIII or FIX as a result of substitution treatment;
- do not use acetylsalicylic acid and nonsteroidal antirheumatic drugs.[1][2]
Von Willebrand disease[edit | edit source]
- The most common congenital bleeding disorder (1-3% of the population); the acquired form also rarely occurs;
- deficiency or dysfunction of the von Willebrand factor (vWF) - i.e. quantitative or qualitative disorder;
- pathophysiology: vWF is formed in vascular endothelium and megakaryocytes; vWF is a glycoprotein that binds to glycoprotein Ib and IIb/IIIa of blood platelets, thereby stimulating their aggregation and adhesion to the damaged vessel wall; vWF is a carrier and stabilizer of FVIII;
- clinical picture: variable bleeding manifestations; often asymptomatic; the most common manifestations are epistaxis, noticeable formation of hematomas , bleeding after an injury in the mouth; heavy menstrual bleeding;
- laboratory examination: APTT prolonged and normal; examination of the level of FVIII and vWF, its antigen (vWF Ag) and functional activity (vWF RCo), examination of ristocetin-induced platelet aggregation (RIPA) and analysis of vWF multimers; genetic tests;
- therapy: mild forms do not require treatment; severe bleeding – antifibrinolytics, desmopressin acetate (increases the level of FVIII/vWF), substitution with plasma concentrate; dispensary in hematology centers.[1]
 For more information see Von Willebrand disease.
For more information see Von Willebrand disease.
Congenital thrombophilic conditions[edit | edit source]
Virchow's triad contributes to thrombosis: 1. damage to the vascular endothelium, 2. slowing of blood flow, 3. imbalance in the blood clotting system.
Leiden mutation[edit | edit source]
- The most common congenital thrombophilic condition (5% of carriers in our population);
- Leiden mutation in factor V gene → resistance to activated protein C;
- AD inheritance; carriers are mostly asymptomatic (they will not experience any thrombosis in their lifetime);
- Significantly increased risk of thrombosis in carriers using hormonal contraception.[1]
 For more information see Leiden mutation.
For more information see Leiden mutation.
Prothrombin mutation[edit | edit source]
- FII; in children, a higher frequency of thrombosis in the CNS area.
Antithrombin deficiency[edit | edit source]
- Tendency to the development of venous thrombosis, already in adolescents and in young adulthood; homozygous form incompatible with life.
Protein C deficiency[edit | edit source]
- Heterozygotes – venous thrombosis in childhood; homozygotes – purpura fulminans; treatment: protein C concentrate.
Protein S deficiency[edit | edit source]
- Venous and arterial thrombosis.
Hyperhomocysteinemia[edit | edit source]
- Venous thrombosis and CNS thrombosis.
Lipoprotein (a)[edit | edit source]
- High levels of lipoprotein (a) - a moderate risk factor for venous thrombosis and ischemic stroke.[1]
Links[edit | edit source]
Related Articles[edit | edit source]
- Disorders of hemostasis : Bleeding conditions (pediatrics) • Hemorrhagic diatheses (pathology) • Acquired coagulopathy • Thrombocytopathy
- Hemostasis • Haemocoagulation • Examination of blood coagulation • Examination of bleeding