Glaucoma
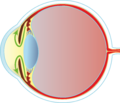
Glaucoma is a group of clinically distinct diseases of various etiologies that cause optic neuropathy and lead to an irreversible impairment of visual function. It is a multifactorial disease, for the development of which increased intraocular pressure is an important risk factor.
Intraocular pressure[edit | edit source]
The intraocular pressure in a healthy eye should be between 9 and 21 mm Hg. Its value depends on the formation and outflow of intraocular fluid. Intraocular fluid is formed in the pars plicata corpus ciliare, flows through the pupil into the anterior chamber and drains through the trabeculae of the anterior chamber angle into the Schlemm's canal, from where it returns to the general circulation through the episcleral and intrascleral veins. To a lesser extent, the intraocular fluid is absorbed by the iris or ciliary body. In glaucoma, the intraocular pressure is increased due to obstruction or impaired absorption of the trabeculae. Increased intraocular pressure causes a decrease in the perfusion pressure of the vessels in the area of the optic nerve papilla. This leads to dysfunction and the death of retinal ganglion cells.
Signs and Symptoms[edit | edit source]
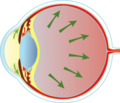
An imbalance in fluid drainage leads to increased intraocular pressure (IOP). This increased pressure pushes on the optic nerve and the retina.
This continuous pressure leads to eventual ‘cupping’ of the nerve, leading to loss of vision. It starts with loss of peripheral vision, and then loss of central vision and eventual blindness.
If the drain remains closed, the pressure accumulates giving the eye the appearance of frosted glass along with intense pain, nausea and vomiting.
Closed-angle attacks are present in the Acute type of Glaucoma. They can occur where the drainage is completely closed off causing spikes in IOP and mild degrees of damage. However, repeated attacks cumulate to cause extensive damage. A sign of this attack is a headache around the eyebrow.
Risk factors for glaucoma[edit | edit source]
- age
- family history
- gender (more often women)
- race (more common in the black population)
- systemic hypertension (after administration of antihypertensives → nocturnal hypotension)
- diabetes mellitus
- refractive errors
- general diseases
- changes in blood clotting and viscosity
Theory of glaucoma[edit | edit source]
- mechanical
- vascular
- neuropathological
Classification of glaucoma[edit | edit source]
- primary glaucoma:
- with an open chamber angle.
- closed chamber angle (acute, malignant, chronic).
- secondary glaucoma:
- open chamber angle (pigmented, exfoliating, post-inflammatory, post-traumatic, postoperative, neovascular).
- with closed chamber angle (post-inflammatory, postoperative, post-traumatic).
- congenital glaucoma:
- early - buftalmus = enlarged bulbus - larger corneal diameter (formation during intrauterine life, bulbus increases due to increased intraocular pressure).
- late - juvenile = mostly congenital occlusion of the outflow tracts at an angle (bulb wall already firm, manifestations as in primary glaucoma).
Primary glaucoma closure glaucoma ( glaucoma angulare )[edit | edit source]
- the intraocular pressure is increased to block the outflow of aqueous humour through the trabeculae of the angle.
- occurs in anatomically predisposed eyes.
- the smaller axial length of the bulb
- the larger radius of the anterior and posterior surfaces of the cornea
- the smaller radius of the front surface of the lens
- larger lens
- forward movement of the lens or free-hanging apparatus
Glaucoma with a closed chamber angle often begins under the picture of recurring so-called small seizures (several hours of headache with iridescence or blurred vision) and then leads to an acute attack of glaucoma.
Acute glaucoma[edit | edit source]
The basic mechanism of acute glaucoma attack (in English terminology acute primary angle-closure) is a complete circular occlusion of the outflow tracts of the iris-corneal angle by the anterior surface of the peripheral part (root) of the iris. With partial occlusion, an acute attack of glaucoma does not occur and the condition may manifest itself in a clinically insignificant increase in intraocular tension (or a so-called small attack accompanied only by a short-term transient cephalea, or with irisation). There are two ways to occlude the iris angle (pulling the iris tissue into the angle during mydriasis and pushing the iris root forward during the pupillary block) and they can complement each other.
1. The peripheral part of the iris can be pulled to an angle, thus blocking the outflow tracts. The most common initial mechanism is a significant dilation of the pupil to 6 mm or more - in the dark, in sleep or under stress. Occlusion is caused by anatomical predisposing factors - a shallow anterior chamber, a narrow iris-corneal angle around 20 °, and the ciliary body configuration forming a plateau iris (slit configuration of the iris-corneal angle) plays a crucial role. Secondly, an acute attack of glaucoma can be induced by an anatomical predisposition to drugs (preparations with a mydriatic effect).
2. The second completely different mechanism is the pushing of the periphery of the iris forward, while again the occlusion of the outflow paths (trabecules) occurs at the iris angle. The essence is the formation of a pupillary block (between the posterior surface of the pupil and the anterior surface of the lens) in the middle mydriasis 4–5 mm on anatomically predisposed eyes. Fluid begins to accumulate in the posterior chamber and pushes the iris root forward, softening the periphery of the anterior chamber until the outflow tracts are occluded by the anterior surface of the iris. An increase in the intraocular pressure of up to 70 torr causes paresis of the pupil sphincter, which leads to mydriasis and exacerbation of angle occlusion. High intraocular pressure reduces eye perfusion, leads to acute retinal ischemia, due to the slow blood flow a thrombus may form resulting in occlusion of the optic nerve papillar arteries and can result in total amaurosis (complete blindness of the eye without light projection) within a few hours of the onset of clinical symptoms. The evoking moment can only be the pronation position of the head, when the lens, due to gravity, rests on the back of the pupil and causes its occlusion - the pupillary block. The closure of the drainage paths at an angle is again conditioned by anatomical factors - the size of the bulb, the free-hanging apparatus of the lens and its size. Secondary the pupillary block occurs when the pupil is occluded or secluded after inflammation or injury, or when the lens swells with age or injury. An acute attack of closed-angle glaucoma with typical clinical manifestations can thus occur even in the originally anatomically completely normal eye. A special form of an acute attack of angle-closure glaucoma is malignant glaucoma with a completely disappeared anterior chamber, arising iatrogenically after operations or after injuries.
Clinical manifestations[edit | edit source]
- headache (irritation n. V from intraocular tension around 30 torrs).
- irrigation: the patient sees rainbow circles around the light (pressure above 30 torrs causes corneal edema and Newton's rings are formed around the point light sources by bending the light).
- impaired, blurred vision (pressure around 50 torrs reduces retinal perfusion and thus its function).
- photophobia (paralysis of the pupil leads to reactive mydriasis).
- nausea and vomiting (reminiscent of a sudden abdominal event) - from parasympathetic irritation - the patient is often admitted to surgery or for persistent headaches due to neurology or infection.
- increased intraocular pressure (up to 70 torrs).
- whitish edematous cornea.
- conjunctival redness.
- more often in women.
Therapy[edit | edit source]
- 1. administer 1% pilocarpine (to narrow the pupil and pull the iris root out of the angle, thus interrupting the occlusion of the outflow tracts) repeatedly after 15 minutes (a more accessible 2% solution in an emergency).
- 2. total drugs to reduce intraocular pressure (osmotic preparations: 50 ml glycerol orally, 100 ml 20% mannitol iv), diuretic Diluran (acetazolamide) orally.
- 3. to improve iris vision and visualization during laser iridotomy, we use a local drop of 20% glucose solution from a vial for i.v. administration.
- topical carbonic anhydrase inhibitors - dorzolamide and brinzolamide (reduce the production of humor aqueous by the ciliary body), beta-blockers - timolol, prostaglandins - latanoprost, travoprost, bimatoprost.
- first choice laser iridotomy to cancel the pupillary block and thus occlusion of the angle.
- or surgical basal iridectomy if the corneal condition does not allow laser surgery (it not only cancels the pupillary block but directly opens the entrance to the outflow tracts).
- malignant glaucoma requires operation in a specialized vitreoretinal workplace.
References[edit | edit source]
Related Articles[edit | edit source]
References[edit | edit source]
- ROZSÍVAL, Pavel. Ophthalmology. 1.. edition. Praha : Galén, 2006. ISBN 80-7262-404-0.
- BOGUSZAKOVÁ, Jarmila – PITROVÁ, Šárka. Acute conditions in ophthalmology. 1.. edition. Praha : Galén, 2006. ISBN 80-7262-368-0.
- HORNOVÁ, Jana. Remedia.cz [online]. [cit. 5.12.2021]. <http://www.remedia.cz/Clanky/Farmakoterapie/Soucasne-moznosti-a-zasady-farmakoterapie-glaukomu/6-L-cK.magarticle.aspx>.