Disorders of purine metabolism
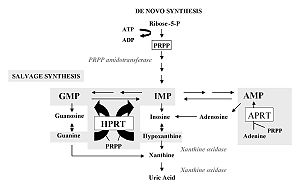
The disorders interfere with both de novo synthesis and salvage pathway reactions and purine catabolism. Purine biosynthesis begins with the formation of PRPP (phosphoribosyl pyrophosphate) catalyzed by the enzyme PRPP synthase. Numerous reactions leading to the formation of IMP (inosine monophosphate) follow. The conservation pathway utilizes food purines that react with PRPP to form nucleotides. IMP is the starting compound for conversions to AMP (adenosine monophosphate) and GMP (guanosine monophosphate) via XMP (xanthine monophosphate). Purine is catabolized via xanthine to uric acid.
Disorders of purine synthesis[edit | edit source]
Increased PRPP synthase activity[edit | edit source]
X-linked inheritance occurs here. Increased PRPP production leads to increased de novo purine production and also to their increased degradation via uric acid. Uric acid is poorly soluble in plasma, increasing its concentration in plasma leads to its crystallization and subsequent settling in soft tissues in the form of sodium urate (Arthritis uratica). The deposits are called gout, they cause an inflammatory reaction, and they are painful. Arthritis uratica may be acute or chronic, urate lithiasis present, and renal failure may occur. Neurological symptoms occur mainly in infants.
Allopurinol (a purine analogue) is used for treatment, which inhibits xanthine oxidase, thereby blocking uric acid production and promoting the accumulation of hypoxanthine and xanthine, which are more soluble. The patient is also recommended a low-purine diet (increased in meat, legumes, coffee, black tea), alkalinity of the indoor environment by sodium bicarbonate and increased fluid intake.
Adenylosuccinate lyase deficiency[edit | edit source]
This is an AR inheritance. The enzyme adenylosuccinase catalyzes two steps in the synthesis of purines, its deficiency causes the accumulation of succinylpurines SAICAR and S-Ado, intermediates of this metabolic pathway in urine and cerebrospinal fluid. We divide this deficit into two types:
- Type I – psychomotor retardation, epilepsy, sometimes muscle atrophy;
- Type II – muscle hypotension, slight delay in psychomotor development, hypotension, convulsions, autism.
For treatment to supplement adenine deficiency in tissues, administration of adenine and allopurinol, which block xanthine oxidase and thus purine degradation, is recommended.
Purine degradation disorders[edit | edit source]
ADA (adenosine deaminase) deficiency[edit | edit source]
This is an inheritance of AR, X-linked. There is an accumulation of adenosine and deoxyadenosine in body fluids. These metabolites are very poorly soluble and form urinary stones. Typical of this disease is high levels of adenine in plasma and severe lymphopenia. Deoxyadenosine and dATP accumulate in lymphocytes, which inhibit the enzyme ribonucleotide reductase required for DNA synthesis (due to this, ADA deficiency belongs to immunodeficiencies as one of the SCID subtypes), ADA activity is also reduced in erythrocytes.ch.
Hypoplasia or absence of lymphatic tissue, variant bone and neurological abnormalities appear. SCID (severe combined immunodeficiency disease) with lymphopenia with frequent infections mainly of the skin, respiratory and gastrointestinal tract is developing. The ADA deficit contributes only 15% to the onset of SCID, in other cases, it develops by other mechanisms.
People without treatment die within two years of life, but at a later age, the disease has a milder course. Clinical signs include mainly pneumonia, candidiasis, chronic watery diarrhea and neurological symptoms (movement disorders and spasticity), with a complication of leukemia.
Increased ADA activity[edit | edit source]
Increased metabolism of ATP and adenine nucleotides in erythrocytes leads to an increase in ADA levels. An approximately 50-fold increase in ADA causes non-spherocytic hemolytic anemia.
Purine nucleoside phosphorylase deficiency[edit | edit source]
It is a disorder of cellular immunity caused by the accumulation of dGTP in T-lymphocytes. This deficit is manifested by anemia, and neurological disorders. There is a marked reduction in uric acid production. It is treated by bone marrow transplantation and transfusion of irradiated erythrocytes.
Xanthine oxidase deficiency[edit | edit source]
This is an AR inheritance. It occurs in two subtypes, the one described being xanthine oxidase / dehydrogenase deficiency. Uric acid is the end product of purine metabolism and is replaced by hypoxanthine and xanthine. This defect causes xanthinuria from which xanthine stones are formed. The isolated xanthine oxidase deficiency is benign, a reduced dietary purine intake (found mainly in meat, legumes, coffee and tea) and an increased drinking regimen are recommended.
Disorders of the salvage pathway[edit | edit source]
Lesch-Nyhan syndrome[edit | edit source]
In this case, it is an X-linked inheritance. An almost complete deficiency of HGPRT (hypoxanthine guanine phosphoribosyltransferase) occurs. This syndrome is one of the most common genetic defects in purine metabolism. It occurs with different symptomatology, both with a partial deficiency of the enzyme, which resembles severe gout in childhood and with urolithiasis and kidney failure.
Accumulation of PRPP (phosphoribosyl pyrophosphate) occurs, which is not used by HGPRT. PRPP is becoming increasingly used for de novo purine synthesis. Increased purine synthesis leads to increased purine degradation. The product of increased purine degradation is uric acid. By increasing the level of uric acid in the serum, uric acid stones are formed.
In the classic form with a complete HGPRT deficiency, children are born clinically healthy. The first symptom is an overproduction of uric acid, whose pink crystals are visible in diapers that have been stained with the newborn's urine. Later, vomiting, convulsions, slow continuous movements, severe psychomotor retardation, and aggression appear. With increasing age, autoutillation (self-harm such as biting lips, fingers, tongue, etc., which cannot be controlled by will) can be observed. Neurological symptoms that appear after 3-4 months are associated with decreased DOPA decarboxylase levels. The relationship between HGPRT and DOPA decarboxylase is not clear. Patients suffer from decreased IQ (50), and dysarthria. The syndrome is treated with allopurinol, a low-urine diet and an increased drinking regime.
In this case, prenatal diagnosis is possible by chorionic villi biopsy or amniotic fluid puncture.
Links[edit | edit source]
Related articles[edit | edit source]
Literature[edit | edit source]
- FERNANDES, John. Diagnostika a léčba dědičných metabolických poruch. 1. vydání. Praha : Triton, 2008. s. 576-580. ISBN 978-80-7387-096-6.
- MURRAY, Robert K., Daryl K. GRANNER a Peter A. MAYES, et al. Harperova biochemie. 4. vydání. Jinočany : H & H, 2002. 872 s. ISBN 80-7319-013-3.
- ŠTERN, Petr, et al. Obecná a klinická biochemie : pro bakalářské obory studia. 2. vydání. Praha : Karolinum, 2011. ISBN 978-802-4619-798.
- ŠEBESTA, Ivan. Poruchy metabolismu purinů [online]. [cit. 2013-07-06]. <http://www1.lf1.cuni.cz/~kocna/biochem/text8.htm>.