Disorders of Purine and Pyrimidine metabolism
This is a group of diseases with neurological, metabolic or immunohematological manifestations, which manifests itself in children and adults.
One of the main causes of these diseases are genetically determined changes in the activity of enzymes . If the activity of the enzyme is reduced or zero, a metabolic block occurs, which leads to the abnormal accumulation of physiological or atypical metabolites in tissues or body fluids and thus leads to damage to the organism. This impairment may also induce increased activity of the enzyme in question, leading to accumulation of metabolites. How the metabolite damages the target organ is currently unknown, so it cannot be effectively intervened in therapy. Targeted treatment is not known especially for effects that damage the CNS.
Most of these diseases have an autosomal recessive mode of inheritance . Only in two diseases is the type of inheritance gonosomally recessive, namely in the defect of HGPRT and PRPP synthase. One of the most common manifestations of purine metabolism disorders is gout, which is defined as a clinical syndrome. More precisely, it is referred to as gouty syndrome, where clinical symptoms only appear in adulthood as painful swelling of the joints. These crystals are released into the joint cavity, where inflammation occurs as a reaction to the IgG coated crystals . This causes the release of inflammatory mediators such as prostaglandins, kinins, histamine . The process is manifested by swelling, redness and great joint pain.
Disorders of Purine metabolism[edit | edit source]
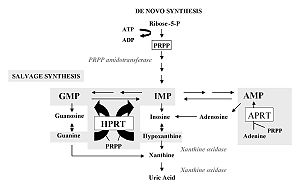
Disorders interfere with the reactions of both de novo synthesis and the salvage pathway and purine catabolism . Purine biosynthesis begins with the formation of PRPP (phosphoribosylpyrophosphate) catalyzed by the enzyme PRPP synthase . Numerous reactions leading to the formation of IMP (inosine monophosphate) follow. The sparing pathway utilizes dietary purines that react with PRPP to form nucleotides. IMP is the starting compound for conversions to AMP (adenosine monophosphate) and GMP (guanosine monophosphate) via XMP (xanthine monophosphate). Catabolism of purines takes place via xanthine to uric acid.
Disorders of Purine synthesis[edit | edit source]
Increased activity of PRPP synthase[edit | edit source]
X-linked inheritance occurs here. Increased formation of PRPP leads to increased de novo formation of purines as well as their increased degradation via the uric acid pathway. Uric acid is poorly soluble in plasma, increasing its concentration in plasma leads to its crystallization and subsequent deposition in soft tissues in the form of sodium urate (Arthritis uratica). The deposits are called gouty tophi, they cause an inflammatory reaction and are painful. Arthritis uratica can be acute or chronic, urate lithiasis is present, kidney failure can occur . Neurological symptoms appear mainly in infants.
For treatment, allopurinol (a purine analog) is administered, which inhibits xanthine oxidase, thereby blocking the formation of uric acid and promoting the accumulation of hypoxanthine and xanthine, which are more soluble. The patient is also recommended a diet with a low purine content (increased in meat, legumes, coffee, black tea) alkalinization of the internal environment by administration of sodium bicarbonate and increased fluid intake.
Adenylosuccinate lyase deficiency[edit | edit source]
This is AR inheritance. The enzyme adenylosuccinase catalyzes two steps in purine synthesis, its deficiency causes the accumulation of succinylpurines SAICAR and S-Ado, intermediates of this metabolic pathway, in urine and cerebrospinal fluid. We divide this deficit into two types:
- Type I – psychomotor retardation, epilepsy , sometimes muscle atrophy ;
- Type II – muscle hypotonia, mild delay in psychomotor development, hypotonia, convulsions, autism.
The administration of adenine and allopurinol, which block xanthine oxidase and thereby the degradation of purines, is recommended for treatment aimed at supplementing the adenine deficit in the tissues.
Disorders of purine degradation[edit | edit source]
ADA (adenosine deaminase) deficiency[edit | edit source]
This is AR, X-linked inheritance. There is an accumulation of adenosine and deoxyadenosine in body fluids. These metabolites are very poorly soluble and form urinary stones. A large amount of adenine in the plasma and significant lymphopenia are typical for this disease. In lymphocytes, deoxyadenosine and dATP accumulate, which inhibits the ribonucleotide reductase enzyme necessary for DNA synthesis (thanks to this fact, ADA deficiency belongs to immunodeficiency as one of the subtypes of SCID), ADA activity is also reduced in erythrocytes.
Hypoplasia or absence of lymphatic tissue, variant bone and neurological deviations appear. SCID (severe combined immunodeficiency disease) develops with lymphopenia with frequent infections mainly of the skin, respiratory and gastrointestinal tract. ADA deficiency contributes to the development of SCID in only 15%, in other cases it develops through other mechanisms.
Those affected without treatment die within two years of life, but at a later age the disease has a milder course. Clinical symptoms mainly include pneumonia, candidiasis, chronic watery diarrhea and neurological symptoms (motility disorders and spasticity). Examples of treatment include bone marrow transplantation, transfusion of irradiated erythrocytes, administration of ADA modified with polyethylene glycol (increased half-life, reduced immunogenicity), successful gene therapy, but with complications of leukemia.
Increased ADA activity[edit | edit source]
Increased metabolism of ATP and adenine nucleotides in erythrocytes leads to an increase in ADA levels. An approximately 50-fold increase in ADA causes nonspherocytic hemolytic anemia
Purine nucleoside phosphorylase deficiency[edit | edit source]
It is a disorder of cellular immunity caused by the accumulation of dGTP in T-lymphocytes. This deficit manifests itself in anaemia and neurological disorders. There is significantly reduced uric acid production. It is treated with a bone marrow transplant and a transfusion of irradiated erythrocytes.
Xanthine oxidase deficiency[edit | edit source]
This is AR inheritance. It occurs in two subtypes, the one described being xanthine oxidase/dehydrogenase deficiency. Uric acid as the end product of purine metabolism is replaced by hypoxanthine and xanthine. This defect causes xanthinuria from which xanthine stones are formed. Isolated xanthine oxidase deficiency is benign , a reduced intake of purines in the diet (found mainly in meat, legumes, coffee and tea) and an increased drinking regime are recommended.
Path-saving malfunctions[edit | edit source]
Lesch-Nyhan syndrome[edit | edit source]
In this case, it is X-linked inheritance. An almost complete deficiency of HGPRT (hypoxanthine guanine phosphoribosyltransferase) appears . This syndrome is among the most common genetic defects of purine metabolism. It occurs with different symptomatology, both with partial enzyme deficiency, which resembles severe gout already in childhood, and with urolithiasis and even kidney failure.
Accumulation of PRPP (phosphoribosyl pyrophosphate) occurs, which is not used by HGPRT. PRPP will be increasingly used for the de novo synthesis of purines. Increased purine synthesis leads to increased purine degradation. The product of increased purine degradation is uric acid. An increase in the level of uric acid in the serum subsequently results in the formation of uric acid stones.
In the classic form with complete HGPRT deficiency, children are born clinically healthy. The first symptom is usually an overproduction of uric acid, whose pink crystals are visible in diapers that have been stained with the newborn's urine. Later, vomiting, convulsions, slow continuous movements, severe psychomotor retardation, aggressiveness appear. With increasing age, self-mutilation (self-harm such as biting the lips, fingers, tongue, etc., which cannot be controlled by will) behavior can be observed. Neurological symptoms appear after 3-4 months and are related to a reduced level of DOPA decarboxylase. The relationship between HGPRT and DOPA decarboxylase is unclear. Patients are affected by a reduced IQ (50), dysarthria. The syndrome is treated with allopurinol, a low-purine diet and increased drinking.
In this case, prenatal diagnosis is possible using a biopsy of the chorionic villi or a puncture of the amniotic fluid
Disorders of pyrimidine metabolism[edit | edit source]
Disorders of pyrimidine metabolism mainly interfere with reactions of de novo synthesis and pyrimidine catabolism . Biosynthesis of pyrimidines takes place in the cytosol. Carbamoyl phosphate reacts with aspartate, gradually forming carbamoylaspartate, dihydroorotate, orotate, OMP (orotidyl monophosphate) and UMP (uridine monophosphate). Catabolism produces β-aminoisobutyrate and β-alanine, which form intermediates of the citric acid cycle .
Disorders of pyrimidine synthesis[edit | edit source]
UMP synthase deficiency (Orotic aciduria)[edit | edit source]
This is AR inheritance. UMP synthase has 2 enzyme activities as orotate phosphoribosyltransferase (OPRT) and orotate decarboxylase (ODC). A block in the synthesis of pyrimidines leads to the accumulation of orotate in body fluids with its increased excretion, crystalluria may occur. At the same time, there is a deficiency of pyrimidines for DNA synthesis resulting in cell division disorders and megaloblastic anemia unresponsive to treatment with iron, vitamin B12 or folic acid, as the disorder lies in insufficient DNA synthesis due to a lack of pyrimidine bases.
Growth disorders, psychomotor retardation, leukopenia, malaise appear. It is treated with uridine. In this case, prenatal diagnosis can also be done.
Disorders of pyrimidine degradation[edit | edit source]
Dihydropyrimidine dehydrogenase (DPD) deficiency[edit | edit source]
This is AR inheritance. Impaired conversion of uracil and thymine to dihydrouracil and dihydrothymine leads to accumulation of uracil and thymine in body fluids. Clinically, complete DPD deficiency appears in children and is accompanied by epilepsy , mental retardation and microcephaly.
The second clinical form is a partial deficiency , which is only discovered in connection with treatment with 5-fluorouracil (tumors), which is not sufficiently degraded (partial DPD deficiency) and is toxic to the patient. It is manifested by neutropenia, stomatitis, neurological symptoms. In connection with neurological symptoms, the reduced production of the neurotransmitter β-alanine, which is a product of pyrimidine catabolism, may be important. Treatment for the pediatric form is not available, in the case of a partial deficiency due to treatment with 5-fluorouracil, we change the chemotherapeutic agent. Even in this case, it is possible to carry out prenatal diagnosis.
Deficiency of dihydropyrimidinase[edit | edit source]
The enzyme dihydropyrimidinase (DHP) cleaves dihydrouracil to β-ureidopropionate and dihydrothymine to β-ureidoisobutyrate. Dihydrouracil, dihydrothymine, less uracil and thymine in urine. Symptoms are similar to DPD deficiency. The treatment is not known.
Thymidine phosphorylase deficiency[edit | edit source]
This deficit was discovered in patients with MNGIE (mitochondrial neurogastrointestinal encephalomyopathy). Accumulation of nucleotides probably leads to mtDNA replication defects .
Clinical symptoms and laboratory findings show lactic acidosis and aciduria and significantly increased excretion of thymidine in urine and blood.
Links[edit | edit source]
References[edit | edit source]
- FERNANDES, John, Jean-Marie SAUDUBRAY, and Georges van den BERGHE, et al. Diagnosis and treatment of hereditary metabolic disorders.1st edition. Prague: Triton, 2008. 607 pp. pp. 576-580. ISBN 978-80-7387-096-6.
- MURRAY, Robert K., Dary K. GRANNER, and Peter A. MAYES, et al. Harper's Biochemistry. 4th edition. Jinočany: H&H, 2002. 872 pp. ISBN 80-7319-013-3 .
- ŠTERN, Petr, et al. General and clinical biochemistry: for bachelor's degree programs. 2nd edition. Prague: Karolinum, 2011. ISBN 978-80-246-1979-8 .
- ŠEBESTA, Ivan. Disorders of purine metabolism [online]. [feeling. 2013-07-06]. < http://www1.lf1.cuni.cz/~kocna/biochem/text8.htm >.
| Hereditary Metabolic Disorders (HMDs) | |
|---|---|
| In general | DMP of complex molecules • DMP of small molecules • Newborn screening • Screening for hereditary diseases • Examination methods for DMP |
| DMP amino acids | Alkaptonuria |
| Organic aciduria | – |
| DMP of the urea cycle | Alkaptonuria • Ornithine transcarbamylase deficiency • Prolidase deficiency • Phenylketonuria • Glutaric aciduria • Hyperphenylalaninemia • Hyperornithineemia • Isovaleric aciduria • Leucinosis • Nonketotic hyperglycinemia • Cystinosis • Tyrosinemia |
| DMP of propionate, biotin
and cobalamin |
Biotinidase deficiency • Methylmalonic acidemia • Propionic acidemia |
| DMP of purines and pyrimidines | Hepatic porphyria • Cutaneous porphyria • Mitochondrial neurogastrointestinal encephalomyopathy |
| DMP sugars | Glycogenoses • Fructoaldolase deficiency • Fructose-1,6-bisphofatase deficiency • Essential fructosuria • Galactokinase deficiency • Galactose-1-phosphate uridyltransferase deficiency |
| Mitochondrial DMP | Phosphoenolcarboxykinase deficiency • LCHAD deficiency • MCAD deficiency • Pyruvate dehydrogenase deficiency • Pyruvate carboxylase deficiency • SCAD deficiency • Chronic progressive external ophthalmoplegia • Leber's hereditary optic neuropathy • Leigh syndrome • Maternal diabetes and deafness • Kearns -Sayre syndrome • VLCAD deficiency |
| DMP of peroxisomes | Neonatal adenodystrophy • Refsum disease • Rhizomelic chondrodystrophia punctata • X-linked adrenoleukodystrophy • Zellweger syndrome |
| DMP of lysosomes | Fabry disease • Gaucher disease • Krabbe disease • Danon disease • Mucolipidosis II • Metachromatic leukodystrophy • Mucopolysaccharidosis III • Niemann-Pick disease • Cystinosis • Tay-Sachs disease |
| Portal: Pathobiochemistry | |