Collision ionization
Collisional ionization is a process where an electron is released after a collision with a particle with high kinetic energy (this energy must exceed the so-called ionization energy – the minimum energy that the incident particle must have in order to tear the electron out of the atomic shell and form a cation ). Collision ionization results in the creation of a positive ion and a negatively charged electron. An electron released in this way can cause the release of additional electrons from other atoms (so-called avalanche ionization).
There are several types of impact ionization – eg ionization by accelerated electrons, by fast molecules (thermal ionization), or by alpha particles.
Collision ionization equation:
Unlike radiant ionization (photoionization), where ionization work (the work required to ionize an atom or gas molecule) is expressed in terms of wavelength, in impact ionization it is expressed in electron volts.
Examples of collisional ionization in practice[edit | edit source]
Collision ionization is encountered, for example, in semiconductors, specifically in so-called avalanche breakdown. When the electric field in a substance is strong enough to accelerate free electrons to such a speed that these electrons have enough energy to knock out a valence electron when they hit the crystal lattice. Here, impact ionization occurs to form an electron-hole pair. Both the colliding and the newly generated electron continue to move and acquire energy that they can use in further collisions. Electrons and holes that are created in the process move in the opposite direction.
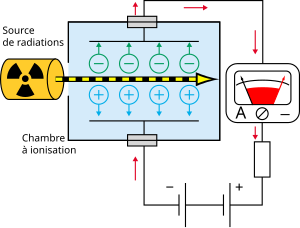
Schematic of the ionization chamber
The ionization chamber consists of two electrodes, between which there is a gaseous medium. These electrodes are well insulated both from each other and from the surrounding environment. The ionization chamber is used to measure ionizing radiation. At the moment when the ionizing radiation reaches the chamber, electrons start to be ejected from the atoms of the non-conductive gas - thus positive ions are created. Positively charged particles move to the cathode, negative particles to the anode.
The volt-ampere characteristic of the ionization chamber can be divided into 3 parts:
- 1. Section [1] of Ohm's law - Formed ions due to the effect of ionizing radiation acquire a small speed, their number does not increase permanently. The current here is directly proportional to the voltage.
- 2. Saturated current section - the section in which the ionization chambers work. The ions acquire a high speed, recombination practically does not occur and practically all ions reach the electrodes.
- 3. Impact ionization section - The ions themselves have such kinetic energy that they are able to ionize neutral gas molecules on their way to the electrodes.
Links[edit | edit source]
Related Articles[edit | edit source]
- Ionization
- https://www.wikiskripta.eu/index.php?title=Z%C3%A1%C5%99iv%C3%A1_ionizace&action=edit&redlink=1
Source[edit | edit source]
- https://en.wikipedia.org/wiki/Impact_ionization
- https://www.wikiskripta.eu/w/Ioniza%C4%8Dn%C3%AD_komora
- wikiskripta.eu/w/Srážková_ionizace
References[edit | edit source]
- Navrátil, Leoš; Rosina, Jozef a kolektiv, Medicínská biofyzika, 1. vyd. Praha: Grada, 2005