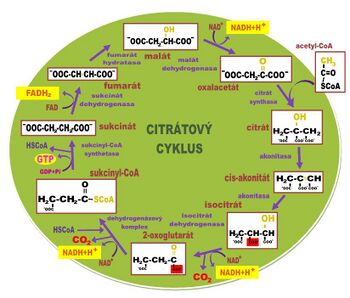
Citrate cycle
Citrate cycle, tricarboxylic acid cycle, TCC, citric acid cycle, Krebs (large) cycle [1][2] is a metabolic pathway of aerobic metabolism (vzniká NADH + H+, is produced , which is consumed in the respiratory chain), although most anaerobic organisms use it to synthesize other metabolites through cata- and anaplerotic reactions (amphibolic nature of TCC, see below, that's why TCC is so complex). In aerobic organisms such as humans, in addition to this, it primarily serves to obtain NADH + H+ and then ATP from pyruvate (product of glycolysis) or acetyl-S·CoA (product of β-oxidation). Enzymes involved in CC reactions are located in the mitochondria except for the succinate dehydrogenase enzyme located in the inner mitochondrial membrane.
An overview of enzymes[edit | edit source]
The pyruvate dehydrogenase complex is a complex of three enzymes inside the mitochondria: pyruvate decarboxylase, dihydrolipoyltransacetylase, and dihydrolipoyldehydrogenase. The complex works as a whole in the presence of coenzymes TPP, NAD+, lipoate in the form of lipoamide, FAD and coenzyme A. Pyruvate dehydrogenase catalyzes the oxidative decarboxylation of pyruvate with the binding of acetyl to TPP, dihydrolipoyltransacetylase catalyzes the transfer of acetyl from TPP via lipoamide to coenzyme A, and dihydrolipoyldehydrogenase regenerates lipoamide using FAD, from which FADH2 is formed, which regenerates in turn by NAD+, from which NADH + H+ is formed. The enzyme is inhibited by arsenic in the oxidation state of As(III) (arsenates,...), which blocks lipoamide.
- 2. Citrate synthase
It catalyzes the transfer of acetyl from acetyl-S·CoA to oxaloacetate with the entry of citrate.
- 3. Aconitase
It catalyzes the conversion of citrate to isocitrate via cis-aconitate. Its active center consists of a cuboidal cluster of four sulfur atoms and four iron atoms, bound via sulfur from cysteine side chains. This enzyme is stereospecific with respect to the prochiral properties of citrate. (This can be demonstrated by labeling pyruvate with carbon 14C). It is inhibited by (2R,3R)2-fluorocitrate.
- 4. Isocitrate dehydrogenase
It carries out oxidative decarboxylation of the carboxyl group on the tertiary carbon of isocitrate while simultaneously dehydrogenating the hydroxy group, changing NAD+ to NADH + H+. This enzyme uses manganese or magnesium ions as a coenzyme.
- 5. α-ketoglutarate dehydrogenase
It catalyzes oxidative decarboxylation with the simultaneous binding of an α-keto carbon to coenzyme A. Thus, sukcinyl-S·CoA and NADH + H+ from NAD+ are formed.
- 6. Succinyl coenzyme A synthetase
It performs the exact opposite reaction of what it is named after. It catalyzes the hydrolysis of succinyl coenzyme A with simultaneous phosphorylation at the substrate level, i.e. GTP is produced directly from GDP (and not in the respiratory chain).
- 7. Succinate dehydrogenase
Performs succinate dehydrogenation. FADH2 is formed from FAD. Dehydrogenation is highly stereospecific and only fumarate and not maleate is formed. The enzyme is competitively inhibited by malonate, which is one carbon shorter than succinate, and therefore dehydrogenation cannot occur.
- 8. Fumarase
It catalyzes the alkaline hydration of fumarate to form malate. OH- carries out a nucleophilic attack on carbon, which has acquired a partial positive charge due to the resonance of π-electrons, thereby introducing a carbanion to which H+ is bound.
- 9. Malate dehydrogenase
It catalyzes the dehydrogenation of malate to oxaloacetate, which regenerates the substrate of the entire cycle. This enzyme may also be involved in the malate shuttle.
Overview of reactions[edit | edit source]
Acetyl-CoA + oxaloacetate[edit | edit source]
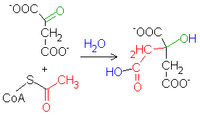 1. Acetyl-CoA + oxalacetate + H2O → citrte + HS-CoA
1. Acetyl-CoA + oxalacetate + H2O → citrte + HS-CoA
- catalyzes the regulatory enzyme citrate synthase
Citrate – isocitrate[edit | edit source]
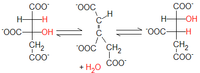 2. Citrate → cis-aconitate + H2O → isocitrate
2. Citrate → cis-aconitate + H2O → isocitrate
- catalyzes aconitase
Isocitrate – 2-oxoglutarate[edit | edit source]
3. isocitrate – 2-oxoglutarate 3. Isocitrate + NAD+(NADP+) → 2-oxoglutarate + NADH+H+(NADPH+H+) + CO2
- is catalyzed by regulatory isocitrate dehydrogenase
2-oxoglutarate – succinyl-CoA[edit | edit source]
 4. 2-oxoglutarate + NAD+ + HS-CoA → succinyl-CoA + NADH+H+ + CO2
4. 2-oxoglutarate + NAD+ + HS-CoA → succinyl-CoA + NADH+H+ + CO2
- catalyzes the regulatory 2-oxoglutarate dehydrogenase complex
Succinyl-CoA – succinate[edit | edit source]
 5. Succinyl-CoA + GDP → succinate + GTP + CoA
5. Succinyl-CoA + GDP → succinate + GTP + CoA
- catalyzed by succinate thiokinase
The macroergic succinyl coenzyme A is hydrolyzed to form succinate and the energy is used for substrate-level phosphorylation. This is an evolutionarily old reaction, which is why guanosine is used instead of adenosine in the conversion of GDP to GTP[3].
Succinate – fumarate[edit | edit source]
6. succinate – fumarate 6. Succinate + FAD → fumarate + FADH2
- catalyzed by succinate dehydrogenase
Fumarate – L-malate[edit | edit source]
L-malate
- catalyzes fumarate hydratase
L-malate – oxaloacetate[edit | edit source]
8. L-malate – oxaloacetate 8. L-malate + NAD+ → oxaloacetate + NADH+H+
oxalacetát + NADH+H+
- catalyzed by malate dehydragenase
The whole cycle is driven by these reactions [3]:
- Decarboxylation of isocitrate — the balance is shifted in favor of α-ketoglutarate as the resulting carbon dioxide is removed from the cells and exhaled.
- Decarboxylation of α-ketoglutarate — the balance is shifted in favor of succinyl coenzyme A — the same as in the previous case.
- Oxidation of malate to oxaloacetate — the balance is shifted in favor of oxaloacetate by the resulting NADH + H+ being consumed in the respiratory chain.
Overview of cataplerotic reactions[edit | edit source]
Cataplerotic reactions are reactions that deplete TCC intermediates.
- Gluconeogenesis;
- Synthesis of fatty acids and cholesterol – starts from acetyl-S·CoA;
- Amino acid synthesis – α-ketoglutarate jis the source for glutamate;
- Synthesis of porphyrins – starts from acetyl-coenzyme A;
- oxidation of amino acids – amino acids can be metabolized into individual TCC intermediates, which are transformed into oxaloacetate, which can be degraded using phosphoenolpyruvate carboxykinase to phosphoenolpyruvate, then to pyruvate, then to acetyl-S·CoA, which is oxidized in TCC to CO2 and carbons from amino acid chains are exhaled.
Overview of anaplerotic reactions[edit | edit source]
Anaplerotic reactions (Greek ana = up, pleroein = to replenish) are reactions that replenish TCC intermediates.
- Oxidation of odd-numbered fatty acids replenishes succinyl-S·CoA.
- Degradation of isoleucine, methionine and valine eplenishes succinyl-S·CoA.
- Transamination and deamination of amino acids leads to the formation of α-ketoglutarate and oxaloacetate.
- Carboxylation of pyruvate - oxaloacetate.
- Oxidative decarboxylation of pyruvate- Acetyl Co A.
Regulation of the citrate cycle[edit | edit source]
Regulation of the Krebs cycle is carried out in several ways:
- enough substances for the course of the cycle, especially Ac-CoA and oxaloacetate.
- elimination of catabolites (see above)
- the cycle is inhibited by an increased ATP:AMP ratio
- respiratory control – the respiratory chain works (the NAD+:NADH+H+ ratio increases)
- enzymatic regulation:
- citrate synthase
- isocitrate dehydrogenase
- α-ketoglutarate dehydrogenase
+++++extras :
Citrate synthase, isocitrate dehydrogenase, and the α-ketoglutarate dehydrogenase complex are enzymes that are allosterically inhibited by high concentrations of ATP. This prevents excessive consumption of acetyl-CoA.
Some enzymesare inhibited if there is a large amount of reduced coenzymes in the mitochondria. The rate of reverse oxidation of coenzymes depends on the respiratory chain, i.e. also on the availability of oxygen. Absence or partial lack of O2 causes complete or partialinhibition of the cycle. On the contrary, the increased rate of respiration, which occurs with an increased need for ATP, also increases the activity of the citrate cycle.
Links[edit | edit source]
Related articles[edit | edit source]
External links[edit | edit source]
Reference[edit | edit source]
- ↑ VOET, Donald – VOET, Judith. Biochemistry. 3rd edition. John Wiley & Sons Inc, 2004. vol. 1616. pp. 765–795. ISBN 0-471-19350-X.
- ↑ NELSON, David L – COX, Michael M. Lehninger Principles of Biochemistry. 4th ed edition. 2004. vol. 1130. pp. 601–623. ISBN 0-71674339-6.
- ↑ a b LIBERDA, Jiří. Citrátový cyklus [lecture for subject Cvičení z biochemie, specialization Chemie v přírodních vědách, Přírodovědecká fakulta UK]. Praha. 7.4.2011.
Used literature[edit | edit source]
- LEDVINA, Miroslav. Biochemie pro studující medicíny. I. díl. 2. edition. Karolinum, 0000. vol. 269. pp. 103-106. ISBN 978-80-246-1416-8.
- MATOUŠ, Bohuslav. Základy lékařské chemie a biochemie. 1. edition. Galén, 2010. vol. 540. ISBN 978-80-7262-702-8.
Recommended literature[edit | edit source]
See references.