Chemical reactions in metabolism
Subchapter content
- The most important chemical reactions in metabolism
- Fundamentals of regulation of metabolic pathways
The most important chemical reactions in metabolism
The metabolic pathways of the human organism form an extensive network of interconnected reactions that often share common intermediates. Chemical transformations of individual substances are usually classified according to a general mechanism common to all substances undergoing a given reaction. For example, decarboxylation is the splitting of CO2 from the carboxyl group, where the substrate may be different carboxylic acids.
Alcohols, carbonyl compounds and carboxylic acids
Alcohols, carbonyl compounds and carboxylic acids are important substrates for many reactions of metabolic pathways of organisms.
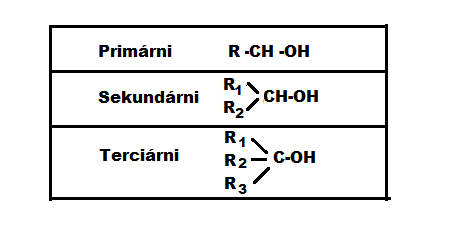
Alcohols contain the functional group −OH. Depending on the number of OH groups in the molecule, alcohols can be one-, two- or polybasic. Furthermore, depending on which carbon atom the OH group binds to, we distinguish between primary, secondary and tertiary alcohols.
Aldehydes with ketones form a group of carbonyl compounds. The functional group of aldehydes is the group −CHO, in ketones −C=O. Of this group of substances, the most important substrates are probably the reactions of carboxylic acids, characterized by the presence of the functional group −COOH, and their derivatives.
Significant reactions of alcohols, aldehydes and carboxylic acids
- Formation of anions and acyls derived from carboxylic acids
- Dehydrogenation and hydrogenation (oxidation and reduction)
- Esterification
Formation of anions and acyls derived from carboxylic acids
The carboxyl group is capable of dissociation, with the degree of dissociation for individual acids given by dissociation constant. Carboxylic acids are weak, which means that their dissociation is only partial. The acid thus gives rise to the anion (group −COO−). After splitting off the whole −OH group from the carboxyl group, its acyl is formed.
Dehydrogenation and hydrogenation (oxidation and reduction)
During the chemical reaction, dehydrogenation, the H is removed from the molecule. The obtained hydrogen can then be used for the formation of a proton gradient in mitochondria and for energy gain (ATP). The introduction of hydrogen into a molecule is called hydrogenation. In the body, dehydrogenation and hydrogenation occur, for example, in the following processes:
- Oxidation of single bonds to double bonds
-
- −CH 2−CH 2− −CH=CH− + 2 H+ +2 e−
- These reactions occur, for example, in Krebs cycle, at β-oxidation of fatty acids or desaturation reactions, which aim at the synthesis of unsaturated fatty acids.
- Mutual conversion of alcohols, aldehydes / ketones and carboxylic acids
- Alcohols, carbonyl compounds and carboxylic acids form a series differing from each other in the degree of oxidation / reduction.
- The general scheme of their mutual transformation is as follows (oxidation takes place towards the carbonyl compound and carboxylic acid, reduction in the opposite direction):
- Primary alcohol aldehyde carboxylic acid
- R−CH2−OH R−CHO R−COOH
- Secondary alcohol ketone
- R 1−CH(OH)−R 2 R1−CO−R2
- Tertiary alcohol
- "Slight" oxidation does not take place (it can be oxidized only with simultaneous splitting of the carbon chain).
An example of oxidation is the formation of dihydroxyacetone phosphate (DHA-P) from glycerol-3-phosphate (cofactor is FAD), through which glycerol enters glycolysis or gluconeogenesis according to the current needs of the organism.
Esterification
Esterification is the reaction of a carboxylic acid with an alcohol, producing an ester and water:
The most important carboxylic acids, their anions and acyls
Saturated monocarboxylic acids
| C | Systematic name | Trivial name | Latin name | Acyl | Anion |
|---|---|---|---|---|---|
| 1 | methane | ant | ac. formicum | formyl | formate |
| 2 | Ethan | Vinegar | ac. aceticum | acetyl | acetate |
| 3 | Propane | propionic | ac. propionicum | propionyl | propionate |
| 4 | Butane | butter | ac. Butyricum | Butyryl | butyrate |
| 5 | pentane | Valérová | ac. Valericum | Valeryl | valerate |
| 12 | Dodecanese | Laurova | ac. Lauricum | Lauryl | laurate |
| 16 | hexadecane | Palmito | ac. palmiticum | Palmitoyl | palmitate |
| 18 | Octadecane | Stearova | ac. stearicum | stearoyl | Stearate |
Saturated dicarboxylic acids
| C | Systematic name | Trivial name | Latin name | Acyl | Anion |
|---|---|---|---|---|---|
| 2 | Etandi | Štavelová | ac. oxalicum | oxalyl | oxalate |
| 3 | Propandiová | Malonova | ac. malonicum | Malonyl | malonate |
| 4 | Butandi | amber | ac. succinicum | succinyl | succinate |
| 5 | Pentondia | glutaric | ac. glutaricum | Glutaryl | glutarate |
| 6 | hexandium | adip | ac. adipicum | Adipoyl | adipate |
Unsaturated monocarboxylic acids
| C | Systematic name | Trivial name | Latin name | Acyl | Anion |
|---|---|---|---|---|---|
| 18:1 | cis-octadec-9-eno | oil | ac. oleicum | oleoyl | oleate |
| 18:2 (ω−6) | cis,cis-octadeka-9,12-dienova | linoleic | ac. linoleicum | Linoloyl | linolate |
| 18:3 (ω−3) | cis,cis,cis-octadeka-9,12,15-trienová | Linolenic | ac. linolenicum | Linolenoyl | Linolenate |
| 20:4 (ω−6) | cis,cis,cis,cis-eikosa-5,8,11,14-tetraenová | arachidonová | ac. arachidonicum | arachidonyl | arachidonát |
Unsaturated dicarboxylic acids
| C | Systematic name | Trivial name | Latin name | Acyl | Anion |
|---|---|---|---|---|---|
| 4 | cis-butendi | Malein | ac. maleicum | Maleinyl | maleate |
| 4 | trans-butendi | Fumarova | ac. fumaricum | Fumaroyl | fumarate |
Carboxylic acid derivatives
| C | Systematic name | Trivial name | Latin name | Acyl | Anion |
|---|---|---|---|---|---|
| 3 | 2-oxopropane | pyrogrape | ac. pyruvicum | Pyruvyl | pyruvate |
| 3 | 2-hydroxypropane | milk | ac. lacticum | lactoyl | lactate |
| 4 | 3-oxobutaneous | acetoacetic | acetoacetyl | acetoacetate | |
| 4 | 3-hydroxybutane | β-hydroxybutyric | β-hydroxybutyrate | ||
| 4 | 2-hydroxybutanedia | apple | ac. malicum | Maloyl | Malate |
| 4 | 2-oxobutandium | oxaloacetic | oxaloacetate | ||
| 5 | 2-oxopentandia | α-ketoglutar | α-ketoglutaryl | α-ketoglutarate | |
| 6 | 2-hydroxypropane-1,2,3-tricarboxylic | lemon | ac. citricum | citrate |
Hydroxy acids and keto acids
Hydroxy acids in addition to the −COOH group also contain the −OH group replacing one −H. Keto acids or oxo acids contain in the molecule in addition to the group −COOH also the group =O replacing one −H. Their mutual conversion is relatively common in metabolic pathways.
An example is the relatively common keto-enol tautomery in metabolism. It converts two forms of organic compounds:
- ketoform (or oxoform) contains double-bonded oxygen as a group =O,
- enolform, which contains a double bond between carbons and one of them binds −OH group (i.e. contains the structure R1−CH=C(OH)−R2).
The mutual transformation of the two forms represents the migration of the hydrogen atom or the proton, accompanied by the swapping of the single bond and the adjacent double bond.
Amino acids and oxo acids
Amino acids and oxo acids are substitution derivatives of carboxylic acids. Amino acids contain in the molecule in addition to the −COOH group also the group −NH 2, oxo acids group =O. Their mutual transformations are frequent in the organism, for example, there is a NH2 group for group =O and vice versa.
These transformations occur mainly in two processes:
- Transamination
- In this reaction, the amino acid is a donor −NH2 of the oxo acid group. From the corresponding oxo acid, an amino acid is formed, and the original amino acid becomes an oxo acid:
- AK1 + OxoK2 OxoK1 + AK2
- Oxidative deamination
- It is the formation of oxo acid from the amino acid by removing −NH2 group, which is released as ammonia (NH3). Oxidative deamination is one of the important reactions through which amino acids initiate the process of their degradation. In the human body, they take place mainly in the liver, and the released ammonia is broken down in urea synthesis.
- This reaction is mainly catalyzed by glutamate dehydrogenase.
Decarboxylation and carboxylation
Decarboxylation removes the carboxyl group, which is released as a CO2 molecule and replaced by a proton. They are significant, for example, for
- conversion of amino acids to biogenic amines (e.g. in the synthesis of many neurotransmitters),
- dehydrogenation of 2-keto acids – pyruvate dehydrogenase reaction and two reactions of the Krebs cycle.
Carboxylation is the opposite reaction, involving the introduction of a COOH group into the molecule. It occurs, for example, in
- fatty acid synthesis,
- gluconeogenesis.
Fundamentals of regulation of metabolic pathways
The regulatory reaction of a specific metabolic pathway is usually localized at its beginning – typically it is the first irreversible step. The reason for this is to limit the waste of resources and the unnecessary production of intermediates that would occur if the track stopped in the middle instead of at its beginning.
The regulatory enzyme is usually present in a low concentration that limits it. It is an "allosteric enzyme" working on the principle of "all or nothing". For regulation, it is advantageous if there is a sort of concentration limit above which the reaction starts and quickly reaches maximum speed, and vice versa, below which the reaction hardly takes place.
The feedback principle is applied in the regulation of metabolic pathways. It is a feedback effect on the course of the reaction from the created intermediates or the final product. We distinguish between two types of feedback:
- negative feedback,
- positive feedback.
Negative Feedback
It leads to a sequence of reactions in which the system returns to its original value. This is the source of stability of the system, which constantly returns to the value of the set point, the set value. Negative feedback is therefore part of most pathways.
Positive Feedback
It leads to a series of reactions that 'deepen it even more. There is, however, a risk of a "vicious circle" (circulus vitiosus). Each further increase in deviation accelerates its increase until finally the instability of the system causes its collapse.
Regulatory step affects
Change in Absolute Enzyme Concentration (Amount of Enzyme)
The process of transcription and translation is affected, namely the induction (activation) or repression (inhibition) of the expression of the gene encoding the given enzyme. An example is substrate induction, where the presence of a substrate induces enzyme synthesis.
Modulation of the activity of an already existing enzyme (enzyme activity)
- the presence of activators / inhibitors,
- covalent modification of the enzyme molecule (phosphorylation / dephosphorylation, formation of active enzymes from proenzymes, ...).
Source
- SVAČINA, Štěpán. Disorders of metabolism and nutrition. 1st edition. Prague: Galén, 2010. 505 pp. ISBN 978-80-7262-676-2 .
- MATOUŠ, Bohuslav, et al. Fundamentals of medical chemistry and biochemistry. 1st edition. Prague: Galén, 2010. 540 pp. ISBN 978-80-7262-702-8 .
- MURRAY, Robert Kincaid, David A BENDER, and Kathleen M BOTHAM, et al. Harper's Illustrated Biochemistry. 5th edition. Prague: Galén, 2012. 730 pp. ISBN 978-80-7262-907-7 .
- LEDVINA, Miroslav, et al. Biochemistry for medical students. 2nd edition. Prague: Karolinum, 2009. 548 pp. ISBN 978-80-246-1414-4 .
- VELÍŠEK, Jan and Jana HAJŠLOVÁ. Food chemistry. 2nd 3rd edition. Tábor: OSSIS, 2009. ISBN 978-80-86659-17-6 .