Breath tests with carbon-13
Modern, non-invasive functional diagnostics in gastroenterology include a number of breath tests. After administration of the tested substrate, exhaled air samples are taken and in 13 C-breath tests the change in the ratio of 13 C and 12 C carbon isotopes in the CO 2 fraction is measured. The 13 C carbon isotope is a marker of the tested function, the administered substrate is enriched with a defined amount of 13 C and this quantity is compared in molar terms with the amount of 13 C isotope in the exhaled air. Older tests used 14 C- radiolabelled substrates, but these tests are in decline and are gradually being replaced by stable 13 C isotope tests.
Determination of ratio 13CO2 : 12CO2[edit | edit source]
There are several methodological approaches to the analysis of exhaled air and the determination of the ratio of 13 CO 2 : 12 CO 2. These appoaches have different principle of detection and analytical sensitivity.
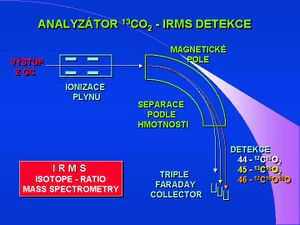
IRMS method[edit | edit source]
The IRMS - Isotope Ratio Mass Spectrometry method is the most accurate, a few microliters of air sample are sufficient for analysis. Special kits are designed for this technique, where exhaled air is collected in 5-10 ml test tubes. The mass spectrophotometer has a special detector, three detectors that capture and count particles with mass 44 ( 12 CO 2 ), mass 45 ( 13 CO 2 ) and mass 46 to eliminate the error in the presence of a stable oxygen isotope - ( 12 C 16/18 O 2 ).
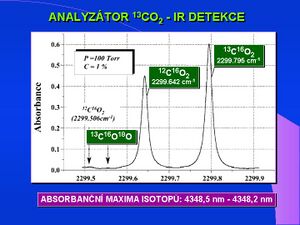
Detection in the infrared spectrum - NDIRS[edit | edit source]
Infrared Detection - NDIRS (Nondispersive Isotope-Selective Infrared Spectroscopy) is the second analytical procedure. The principle of detection is the different absorption maximum of both carbon isotopes in the region of 4350 nm of the infrared spectrum. Analyzers of this type are cheaper, smaller, do not require special operators and are of the POCT (Point Of Care Testing) type, suitable, for example, for specialized clinics. The lower sensitivity of IR analyzers requires much larger air sample volumes (10-100 ml). Aluminum foil bags are used for air collection. The latest technology in the field of IR detection of breath tests is the so-called MCS (Molecular Correlation Spectroscopy) which is based on a pair of very precisely and narrowly specific IR sources, continuous measurement is enabled by a permanent nasal probe. It is a POCT type device.
LARA analyzer[edit | edit source]
The LARA analyzer is the third type of detection. LARA is based on opto-galvanic measurement of laser beam properties, the analyzer of this type is a closed system and is not installed anywhere in our country yet. The accuracy and precision of the breath test results depends not only on the type of analyzer, but above all on the intra- and extra-laboratory quality control and reference sources.
Substrates stable isotope-labeled carbon[edit | edit source]
Currently, there is a wide range of labeled substrates for functional tests of gastric passage ( 13 C-acetate), pancreatic function ( 14 C-cholesteryl octanoid, 13 C-triolein, amylose), intestinal malabsorption ( 14 C / 13 C-xylose, lactose , palmitate), liver function ( 13 C-aminopyrine, phenylalanine, methacetin, leucine, galactose, caffeine), 13 C-urea for urease detection in Helicobacter pylori infection. The testing kits contain a defined amount of substrate, 2 to 6 exhaled air sampling containers (depending on the test layout), and a sampling tube. The pre-analytical phase of the test mainly involves the correct collection of exhaled air, so that the air sample taken in the tube / or aluminum bag contains an air sample with a relatively highest possible CO 2 concentration, the final part of the exhalation, with a concentration corresponding to alveolar air. Screening tests are delivered in a package in which the patient can send them to the laboratory by post after sampling. On-line breath tests are now performed even during endoscopic application of the substrate, most often 13C-urea, and on-line measurement is performed by molecular correlation spectroscopy, NDIRS variant.
Links[edit | edit source]
References[edit | edit source]
- DILLON, EL. Novel noninvasive breath test method for screening individuals at risk for diabetes. Diabetes Care. 2009, vol. 32, no. 3, p. 430-5, ISSN 0149-5992 (Print), 1935-5548 (Electronic). PMID: 19074994.
- GOETZE, O. 13C-methacetin breath test as a quantitative liver function test in patients with chronic hepatitis C infection: continuous automatic molecular correlation spectroscopy compared to isotopic ratio mass spectrometry. Aliment Pharmacol Ther. 2007, vol. 26, no. 2, p. 305-11, ISSN 0269-2813 (Print), 1365-2036 (Electronic). PMID: 17593076.
- MODAK, AS. A simple non-invasive method to detect and monitor hypercapnia: the sodium [13C]bicarbonate breath test. Isotopes Environ Health Stud. 2007, vol. 43, no. 1, p. 23-9, ISSN 1025-6016 (Print), 1477-2639 (Electronic). PMID: 17454270.
- ISHII, Y. Measurement of extra-pancreatic secretory function by 13C-dipeptide breath test. Transl Res. 6, vol. 149, p. 298-303, ISSN 1931-5244 (Print), 1878-1810 (Electronic). PMID: 17543847.
- SLATER, C. Comparison of accuracy and precision of heart rate calibration methods to estimate total carbon dioxide production during 13C-breath tests. Eur J Clin Nutr. 2006, vol. 60, no. 1, p. 69-76, ISSN 0954-3007 (Print) 1476-5640 (Electronic). PMID: 16151459.