Biochemical examination for patient with diabetes mellitus
Disorders of energy metabolism, especially obesity, diabetes mellitus, conditions that precede diabetes (prediabetes) and the so-called metabolic syndrome, have exceptional medical importance. Today, diabetes alone affects around 10% of the population in developed countries and its incidence is still increasing. This is a disease that is associated with a large number of complications and dramatically increases mortality and morbidity. Understanding diabetes and knowing the principles of its diagnosis and treatment is therefore essential for any healthcare professional.
Blood glucose regulation[edit | edit source]
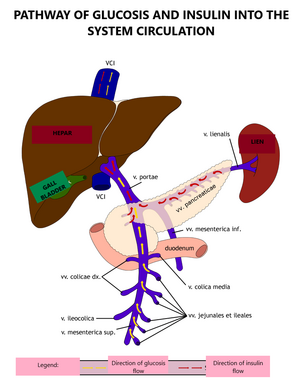
The concentration of glucose in blood (glycemia) under physiological conditions is maintained within a narrow range of values from 3.9 to 5.6 mmol/l in fasting and less than 10 mmol/l after a meal. It is tightly regulated by a number of mechanisms: insulin, which lowers blood glucose, and anti-insulin hormones, glucagon, catecholamines, glucocorticoids, and growth hormone, which increase blood glucose. The liver also plays a significant role in the regulation of glucose homeostasis. Maintaining a constant blood glucose level is essential for the activity of the CNS and other tissues and cells (e.g., erythrocytes).
Glucose sources[edit | edit source]
The exogenous sources of glucose for the body are disaccharides and polysaccharides contained in food. Glucose is formed by their breakdown in the small intestine and is utilized in the liver, muscles, adipose and brain tissue as a direct source of energy. Unoxidized glucose is stored in the form of glycogen or converted to fatty acids and triacylglycerols. In the fasting state, the normal glucose concentration is maintained by the breakdown of glycogen by glycogenolysis and the formation of glucose from non-saccharide precursors (amino acids, glycerol and lactate) in the process of gluconeogenesis.
Changes in blood glucose level[edit | edit source]
- A fall in blood glucose below 3.2 mmol/l is called hypoglycemia. In hypoglycemia, the supply of glucose to brain tissue is compromised. It can occur during various diseases, most often in overdose of antidiabetics, more rarely in long-term starvation, in some endocrine tumors or in inherited metabolic disorders that also affect glucose metabolism (eg glycogenosis). Severe hypoglycaemia is accompanied by restlessness, sweating and tremor.
- Glycaemia elevated above the reference range is referred to as hyperglycemia. Chronic hyperglycemia is a basic manifestation of diabetes mellitus. However, we may also encounter transient non-diabetic hyperglycaemia. All the situations where there are elevated levels of catecholamines, glucocorticoids and other stress hormones, including a number of acute diseases, lead to it. A change in the regulation of glucose metabolism leading to hyperglycemia also accompanies inflammatory conditions. Hyperglycemia can also be caused iatrogenically, most often as a side effect of treatment with steroid hormones and their analogues.
Diabetes mellitus[edit | edit source]
Diabetes mellitus (DM) is a chronic metabolic disease with high morbidity and mortality, the main and common manifestation of which is hyperglycemia due to absolute or relative insulin deficiency. There has been a significant and steady increase in the last decade, and there is also a talk of a diabetes epidemic or pandemic. The cause of this phenomenon lies in the lifestyle of the population of economically developed countries (excessive energy intake, reduced physical activity).
At present, more than 9% of people with diabetes are registered in the Czech Republic (990,000). Another 5% of the population is expected to remain undiagnosed. These are most often type 2 DM (93% of cases), type 1 DM makes up about 5%, and other types are rather rare. [1]
Classification[edit | edit source]
Diabetes mellitus[edit | edit source]
- Type 1 diabetes mellitus
- autoimmune,
- type LADA (develops at a later age, it is often confused with DM type 2),
- idiopathic.
- Type 2 diabetes mellitus
- with a predominant impaired insulin secretion,
- with predominant insulin impairment.
- Other specific types of DM
- pancreatopriv DM (chronic pancreatitis, resection, trauma, cystic fibrosis, hemochromatosis),
- DM in endocrinopathies (acromegaly, glucagon, pheochromocytoma, hypercortisolism, hyperthyroidism, etc.),
- Drug- and chemical-induced DM (glucocorticoids, nicotinic acid, thyroid hormones),
- monogenic forms (MODY).
- Gestational diabetes mellitus.
Prediabetes[edit | edit source]
- Increased fasting glucose (IFG).
- Impaired glucose tolerance (IGT).
- For more information, see the Prediabetes page .
Pathogenesis[edit | edit source]
Disruption of glucose metabolism occurs:
- impaired insulin production or secretion (insulin deficiency),
- insulin disorder (insulin resistance),
- possibly a combination of both mechanisms.
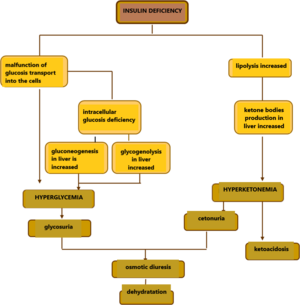
Insufficient insulin function disrupts the transport of glucose from the blood to the cell, leading to hyperglycemia and glucose deficiency intracellularly. Insufficient glucose utilization leads to a change in the mechanisms for ATP gain. Gluconeogenesis and glycolysis are stimulated, and lipolytic breakdown of triacylglycerols into fatty acids and glycerol increases in adipocytes. Degradation of fatty acids by β-oxidation produces excess acetyl-CoA, from which ketone bodies (acetacetate, 3-hydroxybutyrate and acetone) are formed in the liver. Acetacetate can serve as a source of energy for muscle and brain function instead of glucose.
If the production of ketone bodies exceeds their utilization by peripheral tissues, ketoacidosis develops. Because ketone bodies are soluble in water and excreted in the urine, ketonuria occurs. When the threshold plasma glucose concentration (10-12 mmol/l)[2] is exceeded, the transport capacity of the proximal tubule is disrupted and glucose enters the final urine. Glucose and ketone bodies are osmotically active, so they entrain even more water into the urine, which is the basis of polyuria .
Clinical picture[edit | edit source]
From the above, the characteristic symptoms of DM, such as thirst and polydipsia, osmotic polyuria, inconstantly present anorexia and weight loss caused by fluid loss, decreased food intake and a negative energy balance in case of large urinary glucose losses, result.
Psychological aspects of treatment[edit | edit source]
Due to the need for comprehensive treatment of the disease, cooperation at the doctor-patient level is important. A patient with diabetes may experience problems associated with a new diagnosis, necessary precautions, or related secondary complications that may lead to diabetic distress. It is appropriate for the doctor to focus on the psychological aspects of this disease.
Complication[edit | edit source]
- Acute complications
- Hypoglycemia,
- diabetic hyperosmolar hyperglycemic coma,
- diabetic ketoacidosis
- ketoacidotic coma
- lactic acidosis,
- lactacidotic coma.
- Late complications
- Diabetic kidney disease
- diabetic retinopathy,
- diabetic neuropathy,
- diabetic foot syndrome,
- atherosclerosis.
- For more information, see Diabetes Mellitus Complications.
Type 1 diabetes mellitus[edit | edit source]
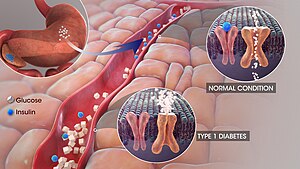
Type 1 diabetes mellitus is characterized by an absolute or almost complete deficiency of endogenous insulin and a life dependence on the administration of exogenous insulin. Patients are prone to ketoacidosis.
The disease results from selective destruction of β cells of Langerhans' islets of the pancreas by an autoimmune process in the genetically predisposed individuals. The triggering mechanism of the autoimmune process is probably a viral infection or contact with another exogenous or endogenous agent.
The clinical picture of type 1 diabetes mellitus depends on the aggressiveness of the autoimmune process. In childhood and adolescence, when most diseases develop, the last stage of β-cell destruction tends to be very rapid, so diabetes is manifested by classic acute symptoms (including ketoacidosis). At a later age, the disease tends to have a much slower onset and only eventually results in the complete insulin dependence. Insulin secretion may be reduced for several years, but sufficient to prevent ketoacidosis. The clinical course of the disease therefore resembles type 2 diabetes mellitus, and it is stated that about one in ten patients originally classified as type 2 diabetes has the slow-onset type 1 diabetes – latent autoimmune diabetes adult (latent autoimmune diabetes of adults – LADA).[3]
Type 1 DM is a less common form of diabetes that occurs in about 7% of diabetics. The classic symptoms of type 1 DM are thirst, polyuria and weight loss.
| Type 1 diabetes mellitus | Type 2 diabetes mellitus | |
|---|---|---|
| LADA | ||
| insulin secretion is missing | gradual cessation of insulin secretion | insulin resistance, impaired insulin secretion |
| a typical beginning in childhood and adolescence | a typical beginning in adulthood | a typical beginning after 40 years |
| ketoacidosis | ||
| more often lower BMI | more often higher BMI | |
| positive autoantibodies | autoantibodies are missing | |
| C-peptide is missing | C-peptide reduced | C-peptide normal or elevated |
| immunoreactive insulin is missing | immunoreactive insulin reduced | immunoreactive insulin normal or elevated |
Type 2 diabetes mellitus[edit | edit source]
Type 2 is the predominant form of DM. Patients are not vitally dependent on exogenous insulin because insulin production is not reduced, or is reduced less than in type 1 DM.
The cause of this type is a dysfunction in the action of insulin. This is called insulin resistance due to a disorder of the insulin receptor or a disorder in the transmission of the insulin signal to the cell.
The concentration of insulin in the blood tends to be initially, due to insulin resistance, increased. In the further course of the disease, a disorder of insulin secretion also occurs, β-cells gradually lose their ability to respond to increased glucose levels by insulin synthesis.
The disease manifests itself mainly in adulthood, usually over the age of 40. Type 2 DM has a high heredity, so the family burden is evident in the anamnesis. Unlike type 1, patients are not prone to ketoacidosis. 60-90% of cases are associated with obesity.
Gestational diabetes[edit | edit source]
Gestational diabetes mellitus (GDM) is a disorder of various stages of glucose metabolism that occurs during pregnancy and resolves spontaneously during the sixth trimester. In addition to GDM, the so-called overt diabetes mellitus (DM) can also be detected during pregnancy, it meets the diagnostic criteria of diabetes valid for the general population and usually persists after the sixth week. Caring for pregnant women with overt diabetes is the same as caring for pregnant women with pregestational diabetes.
All pregnant women, except those already treated for diabetes, are subjected to a two-phase screening of GDM provided by an outpatient gynecologist. In the first trimester of pregnancy (up to the 14th week), fasting venous blood glucose is determined. If a pregnant woman has fasting blood glucose repeatedly (ie 2 consecutive days) ≥ 5.1 mmol/l, she is diagnosed with GDM, no longer has to undergo oral glucose tolerance test (oGGT) and is referred to a diabetologist. All women with a negative result in the 1st trimester then undergo a three-point oGGT with a load of 75 g of glucose between 23rd + 1 to 27th+ 6 weeks of pregnancy if they have a fasting blood glucose lower than 5.1 mmol/l. Normal glycemias in oGTT are < 10.0 l mmol/l at 60th minute and < 8.5 mmol/l at 120th minute. At higher values, GDM is diagnosed and the woman is referred to diabetology. Depending on the severity of the GDM, diet, metformin or insulin are used to compensate for blood glucose[4].
Borderline disorders of glycoregulation[edit | edit source]
The following are considered to be the boundaries between normal glucose tolerance and diabetes mellitus:
Basic biochemical examinations in patients with diabetes mellitus[edit | edit source]
Determination of blood glucose concentration is an examination that will provide basic information about carbohydrate metabolism. Capillary or venous blood is collected and glucose is determined in whole blood, plasma or serum. When determining glucose in whole blood, the values are 10–15% lower (depending on the hematocrite), in arterial blood they are 10% higher than in venous blood (arteriovenous difference). NaF (2.5 mg per ml of whole blood) is added to the collection vials to prevent glycolysis .
Blood glucose testing has the necessary informative value only if the time interval between blood collection and a food intake is known.
Blood glucose testing is performed:
- fasting (blood is taken at least 8 hours after food intake) - indicated when searching for diabetics and diagnosing DM;
- randomly measured glycemia (blood is taken without indicating the time relationship to food intake) - is performed when hypoglycemia or hyperglycemia is suspected;
- postprandial glycaemia (1 hour after a carbohydrate meal) - indicated when monitoring the effectiveness of DM treatment;
- as a glycemic profile - blood glucose is determined several times a day, usually before main meals, sometimes after meals and at night.
Methods for determining blood glucose[edit | edit source]
Determination of blood glucose in laboratory conditions[edit | edit source]
Various methods are used to determine glucose concentration. Enzyme methods are widespread. Glucose can be determined by any enzyme that metabolizes it. Another article discusses the possibilities of non-invasive blood glucose measurement
Glucose oxidase reaction[edit | edit source]
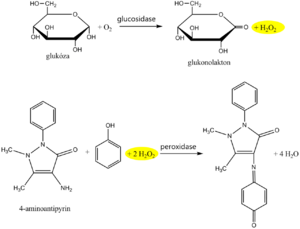
The recommended routine method uses coupled enzymatic reactions of glucose oxidase (GOD, EC 1.1.3.4) and peroxidase (POD, EC 1.11.1.7). In the first reaction, the enzyme glucose oxidase catalyzes the oxidation of glucose by atmospheric oxygen to form gluconic acid, which is converted to the internal ester gluconolactone. It is known that 36% of glucose is in the form of the α-anomer and 64% in the form of the β-anomer. GOD is highly specific for β-D-glucopyranose. In order for both anomers to be oxidized, a mutation of the α- to β-anomer is required, which occurs spontaneously during a sufficiently long incubation. An equimolar amount is formed as a by-product of the glucose oxidase reaction hydrogen peroxide.
In another peroxidase-catalyzed reaction, the hydrogen peroxide formed reacts with a suitable chromogen, which is oxidized to a reactive intermediate, which is coupled with another substance to form a stable soluble dye. An example is the oxidative coupling of a phenol derivative with 4-aminoantipyrine to a red dye, the absorbance of which is measured after the reaction equilibrium has stabilized.
Other methods use measuring the loss of oxygen that occurs during a glucose oxidase-catalyzed reaction, which can be monitored electrochemically with an oxygen electrode or an enzyme electrode.
Hexokinase reaction[edit | edit source]
The hexokinase method is characterized by high specificity. Hexokinase (EC 2.7.1.1) phosphorylates glucose in the presence of ATP to form glucose-6-phosphate. In the next step, glucose-6-phosphate is oxidized by glucose-6-phosphate dehydrogenase against NADP+ to 6-phosphogluconolactone. The reduction of NADP+ to NADPH can be evaluated by direct photometry in the UV region on the principle of Warburg optical test.
Determination of glycemia in non-laboratory conditions[edit | edit source]
Glycemia is one of the parameters that is often examined even without a laboratory background. Rapid guideline blood glucose determination is common in emergency care. In patients treated with insulin, it is also preferable to monitor glycemia regularly with a personal glucometer with treatment adjusted based on the measured values. Blood glucose concentration is among the parameters that are most often determined by the techniques of screening at the site of patient care (point of care testing , POCT). However, it must be borne in mind that POCT methods, although improving the quality of care and patient comfort, do not replace regular medical examinations or laboratory tests.
Rapid blood glucose methods use several principles. The starting material is usually a drop of full capillary blood which is applied to the test strip.
The oldest bands were based on the same reactions as the photometric measurement of glucose concentration. The reaction zone contained glucose oxidase, peroxidase and the appropriate chromogen. The evaluation was performed either visually, by comparison with a color scale, or using a glucometer - a single-purpose reflection photometer.
Most glucometers today use enzyme electrodes. [5] [6]
The first generation of sensors appeared in the 1960s. The oldest system was based on the glucose oxidase reaction. It used two electrodes, one covered with an enzyme. The concentration of oxygen in the sample and the rate of its decrease during the reaction was measured by the so-called Clark method: oxygen is reduced at the platinum cathode, the current intensity between the cathode and the anode corresponds to its concentration:
- O2 + 4 H+ + 4 e- → 2 H2O
Later, hydrogen peroxide production was determined electrochemically instead of oxygen consumption. Even in this case, it is a simple electrochemical reaction, this time taking place at the anode:
- H2O2 → O2 + 2 H+ + 2 e-
- The analyzers constructed in this way were simpler and could be made smaller. However, amperometric measurement of hydrogen peroxide production is influenced by a number of substances: ascorbate, uric acid, many drugs, etc. Another problem of many first-generation sensors was the dependence of the measurement results on the oxygen saturation of the sample.
- Second-generation sensors are also based on the glucose oxidase reaction, but instead of molecular oxygen, the electron acceptor is supplied by another substance - the so-called mediator. Another possibility is the oxidation of glucose to gluconolactone by another bacterial enzyme, glucose dehydrogenase, whereby electrons are again transferred to a suitable mediator. In both cases, the reduced mediator is reoxidized at the anode and either the current flowing between the cathode and the anode (amperometric determination) or the resulting anode charge (coulumbometric determination) is measured. A number of specific test strip configurations are used, and various substances play role of mediators (eg, ferric cyanoferrate, ruthenium hexamine, osmium complexes, phenanthrolinequinone).
Oral glucose tolerance test[edit | edit source]
The oral glucose tolerance test (oGTT) reflects the body's response to glucose in a physiological way and assesses whether the body is able to maintain blood glucose levels within the normal range after glucose exposure. It is a summary of information not only about the effects of hormones regulating glycemia, but also about processes in the gastrointestinal tract (rate of gastric emptying, intestinal passage and resorption) and liver function.
The oral glucose tolerance test is used primarily for the early diagnosis of gestational diabetes. In this case, it is used as a routine screening examination, performed on all pregnant women at the turn of the second and third trimesters (or as soon as possible for high-risk pregnancy).
In others, the oral glucose tolerance test is recommended as an additional diagnostic test for the diagnosis of DM if fasting and random glucose values cannot be determined (especially if fasting blood glucose is between 5.6–7.0 mmol/l).[7] If fasting or random blood glucose levels are conclusive for the diagnosis of DM, oGTT would be an unnecessary burden for the patient and is therefore contraindicated. Furthermore, it is not performed in acutely ill and immobilized patients or in patients on a reduction diet.
Execution of oGTT[edit | edit source]
- 3 days before the test we do not limit the intake of carbohydrates (at least 150 g/day) and the patient performs the usual physical effort;
- after fasting for 8-14 hours, a fasting blood sample is taken;
- the patient drinks 75 g of glucose dissolved in 250–300 ml of tea or water within 5–10 minutes;
- the patient remains seated and does not smoke during the examination;
- another blood sample is taken in pregnant women at 60th and 120th minute, in all other patients only at 120th minute after glucose exposure.[8]
In addition to the above-mentioned oGTT method, an even more detailed so-called glycemic curve can be performed, where blood is taken several times, usually at 30-minute intervals.
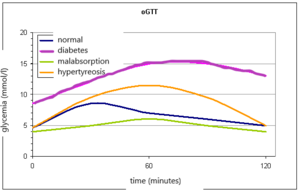
We can describe three sections of the graph of the glycemic curve:
- Glucose is, after oral administration, absorbed in the intestine and blood glucose increases - rising part. It is usually steep after gastrectomy and flat during malabsorption. As the blood glucose increases, the oxidation of glucose in the muscles is stimulated and glycogen is formed in the liver.
- The function of the liver and the effects of insulin in the liver are reflected by next section - the peak part of the glycemic curve. In the onset of DM, glucose in the liver is not sufficiently converted to glycogen, and therefore the peak of the glycemic curve exceeds 11.1 mmol/l and the maximum occurs later than in 60 minutes. In liver disease, the apex of the curve is also altered. Hepatocytes are not enough to metabolize the absorbed glucose, so more glucose passes into the periphery. The peak also exceeds 11.1 mmol/l and the increase persists for more than 60 minutes, but unlike DM, it returns to normal at 120 minutes (bell shape of the curve). In hyperthyroidism, the level of 11.1 mmol/l is also exceeded due to rapid absorption, but the decrease is rapid (Gothic curve).
- The descending part is dependent on the effects of insulin and is physiologically characterized by a decrease in glucose levels. A slow and insufficient return of blood glucose to normal is a classic manifestation of DM.
Normally, we observe a maximum rise in 30–60 minutes, there is no glycosuria, and after 2 hours the blood glucose returns to fasting values.
In general, the oGTT is burdened with a large random error and low reproducibility.
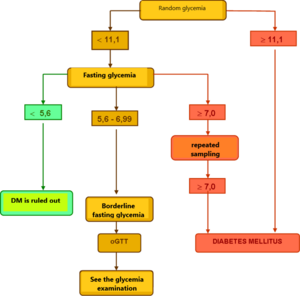
Evaluation of glycemia and oGTT[edit | edit source]
Healthy individuals have fasting plasma glucose < 5.6 mmol/l, with oGTT having a fasting blood glucose < 7.8 mmol/l at 120 minutes. The diagnosis of DM is testified by
- fasting blood glucose > 7.0 mmol/l or
- random blood glucose > 11.1 mmol/l or
- glycemia >11.1 mmol/l at 120 minutes oGTT
together with classic clinical symptoms (thirst, polyuria, weight loss).
To confirm the diagnosis of DM, a repeated examination from the next collection is required on one of the following days.
Glycated proteins[edit | edit source]
A direct consequence of elevated blood sugar levels is its intense binding to proteins, which is the essence of non-enzymatic glycation. The glycation process is conditioned by the presence of free amino groups in the protein molecule.
The rate of glycation is mainly influenced by the concentration of glucose and its duration of action. It also takes place in healthy individuals. Glycation takes place on all serum proteins and leads to the formation of glycated derivatives. Both soluble and structural proteins are glycated.
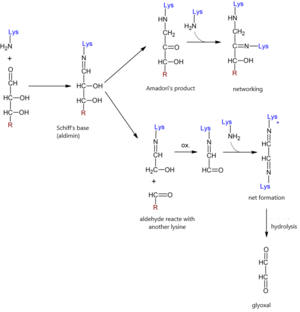
Non-enzymatic glycation takes place in several phases of the so-called Maillard reaction.
- The sequence of glycation reactions is initiated by the aldehyde group of the reducing sugar, which binds to the amino group of the protein. A labile aldimine of the Schiff base type (early glycation products) is formed. Schiff's formation is quick, reaching equilibrium within hours. This reaction is reversible.
- Schiff's base then slowly, over several days, undergoes chemical rearrangement. A more stable glycation transition product is formed, the so-called Amadori product, which has the character of a ketoamine. This produces a keto group on the second carbon of sugar, which is characteristic of fructose. Concentration resulting fructosamines can determine responses to nitroblue tetrazolium (see below). Amadori's products are also to some extent reversible, although the balance is significantly shifted in the direction of their formation. Steady state is reached in a few weeks. The amount of ketoamines can be reduced by normalizing blood glucose.
- Aldose, which is two carbons shorter than the original protein-bound sugar, can also be cleaved from Schiff's base. A di-carbon residue remains attached to the protein, which is further oxidized and reacts with another amino group of the protein. The chain of further reactions results in the formation of highly reactive glyoxal-type dialdehydes.
- Amadori products are reactive substances. Over the months and weeks, irrespective of the presence of glucose, the advanced glycation end products (AGEs) result from the reaction of the Amadori products primarily with long-lived proteins (collagen, elastin, myelin nerve). These processes are irreversible.
Non-enzymatic glycation in long-term hyperglycemia is one of the causes of tissue damage in some organs in patients with diabetes mellitus.
The determination of Amadori-type glycation products is a suitable indicator of long-term glucose concentration and provides indirect information on the course of glycaemia over a period of time that corresponds to the biological half-life of the protein. Glycated hemoglobin and glycated proteins are routinely determined.
The determination of glycated hemoglobin and glycated proteins is used to control diabetes mellitus and to diagnose persistent hyperglycemia. Elevated glycated derivatives indicate that elevated blood glucose levels have prevailed in the patient in recent weeks and that diabetes mellitus has not been adequately controlled.
Glycated hemoglobin[edit | edit source]
Glycated hemoglobin (HbA1c) is considered the best way to control glucose levels in diabetics over the long term. Glycated hemoglobin concentration indicates blood glucose values in the previous 2–3 months (erythrocyte lifespan). It is determined by chromatographic or immunochemical methods.
Evaluation of glycated hemoglobin (HbA1c) levels in diabetics
| Compensated DM | ≤ 45 mmol/mol |
| The need for a change in therapy | ≥ 53 to 70 mmol/mol |
Glycated hemoglobin concentration is measured with respect to the total hemoglobin concentration and is expressed in mmol HbA1c per mole of total hemoglobin. An expression in percent of HbA1c in the total hemoglobin (1% ≙ 10 mmol/mol) can also be found. The assessment of glycated hemoglobin concentration in the table above is indicative. Target concentrations vary according to the risk of hyper-/hypoglycemia in a particular patient.[9]
The glycated hemoglobin assay can also be used to screen for diabetes mellitus. For HbA1c concentrations above 39 mmol/mol, diabetes mellitus is suspected.[10]
If the concentration of HbA1c is higher than 48 mmol/mol, the diagnosis of diabetes mellitus can be considered confirmed.[11]
Evaluation of glycated hemoglobin (HbA1c) levels in diabetes mellitus screening
Physiological values < 39 mmol/mol Suspicion of diabetes mellitus 39–48 mmol/mol Diabetes mellitus ≥ 48 mmol/mol
Fructosamines[edit | edit source]
Glycated proteins or fructosamines have a shorter half-life and their level reflects the average glucose concentration for the period of 2–3 weeks before the examination. Their main component is glycated albumin. Hypoproteinemia may falsely reduce the results. Today, determination of fructosamine concentrations is not a routine test in diabetics.
| Physiological values: | 205–285 μmol/l |
| Good DM compensation: | 285–320 μmol/l |
| Satisfactory DM compensation: | 321–370 μmol/l |
| Bad DM compensation: | above 370 μmol/l |
Principle of determination of glycated proteins (fructosamine)[edit | edit source]
Examination of glycated proteins uses the reducing properties of fructosamine in an alkaline environment. In the presence of carbonate buffer, fructosamine rearranges to its tautomer - eneaminol, reacts with nitrotetrazolium blue (NBT). During the reduction, the heterocyclic rings of NBT are opened and colored formazan is formed. The rate of formazan formation is directly proportional to the fructosamine concentration.
Like any method that utilizes the reducing properties of some serum components, this test is non-specific. It therefore begins with a few minutes of incubation to remove the effect of fast-reacting reducing agents. In commercial kits, the agent includes the enzyme uricase, which eliminates the reduction of NBT by uric acid.
Glucose in urine[edit | edit source]
Glucose filtered into the primary urine from the plasma is reabsorbed by active transport in the proximal tubules. The body protects glucose from unnecessary losses, so the renal tubules have a considerable reserve capacity - under physiological circumstances, the transport system uses only about one third of its capacity. In hyperglycemia, the utilization of the transport system increases, and if the glycemia exceeds about 10 mmol/l (called renal threshold for glucose), the capacity of tubular resorption is exceeded and glucose passes into the urine. Urinary glucose losses greater than 0.72 mmol/24 hours are referred to as glycosuria (glucosuria). Glycosuria is the most common finding leading to the detection of diabetes mellitus. However, a negative finding of glucose in the urine does not rule out this disease. Therefore, the determination of glucose in urine is not one of the basic biochemical parameters used for the diagnosis and monitoring of DM.
The finding of glycosuria should be evaluated together with the level of fasting blood glucose. Based on blood glucose, we distinguish:
- Hyperglycemic glycosuria, which is a typical finding in diabetes mellitus. However, with prolonged illness, the renal threshold for glucose increases and glycosuria may even disappear. Therefore, it is only a cursory examination, on the basis of which the DM therapy cannot be managed. So-called "foodborne glycosuria" may also occur temporarily as a result of a carbohydrate-rich diet or during oGTT.
- Normoglycaemic renal glycosuria, in which blood glucose levels are not elevated. It is the result of a disorder in the kidney tubular cells, which ensure the resorption of glucose. It can be a manifestation of an autosomal recessive disease, but it occurs more often, for example, in toxic or inflammatory kidney damage affecting the function of the proximal tubule.
Methods for determining glycosuria[edit | edit source]
Non-specific chemical reactions or test strips can be used to determine glycosuria.
Non-specific chemical reactions are based on the reducing properties of monosaccharides. Fehling's and Benedict's tests use non-specific reductions of the CuII+ chelate complex with the citrate or tartrate to Cu1+. In the Nylander's test, bismuth nitrate BiNO3(O) is reduced to black metallic bismuth in the presence of reducing agents. These reactions are positive not only for glucose, but also for other reducing carbohydrates, or substances with reducing properties (ascorbic acid). The urine tested must not contain proteins.
Determination of glucose in urine with a diagnostic strip[edit | edit source]
Diagnostic strips for the detection of glucose in urine are based on the principle of enzymatic reactions with glucose oxidase and peroxidase (same principle as the determination of glycemia). D-glucose is oxidized using glucose oxidase and oxygen is formed D-glukono-1,5-lactone and hydrogen peroxide. In a subsequent peroxidase reaction, hydrogen peroxide oxidizes tetramethylbenzidine or another chromogen to a colored product. The light yellow color of the reaction surface changes to blue-green when positive. The test is specific for D-glucose, other sugars do not give a positive reaction.
High concentrations of reducing agents such as ascorbic acid slow down the development of color and can lead to falsely lower results. In these cases, it is recommended to repeat the analysis at least 10 hours after a vitamin C withdrawal. Conversely, false positive results may be due to the presence of peroxidase substrates or oxidizing agents in the sampling vessel (for example, H2O2, Persteril®, chloramine B). Urine glucose determination should be performed rapidly to avoid bacterial contamination, or the urine should be stored at 4 ° C.
Interference with ascorbic acid is a common source of false negatives. Urine test strips from some manufacturers are therefore modified so that the reaction zone is at least somewhat resistant to ascorbic acid. Some diagnostic strips also have an ascorbate detection zone to alert you to false negatives.
Positive glycosuria is an urgent indication for fasting blood glucose testing.
Ketone bodies in urine[edit | edit source]
By "ketone bodies" we mean acetoacetic acid, acetone and β-hydroxybutyric acid. Increased excretion of ketone bodies in urine indicates increased lipolysis and increased formation of acetyl-CoA, which is due to insufficient utilization of glucose in the absence of insulin not normally metabolized in the Krebs cycle. The result is the accumulation of acetyl-CoA, which condenses to acetoacetic acid. Most of the acetoacetic acid is reduced to β-hydroxybutyric acid in the liver, a smaller part spontaneously decarboxylates to acetone.[12]
Determination of ketone bodies in urine is important especially in type 1 diabetics. We do not find ketone bodies in the urine of a properly treated diabetic. Their presence indicates metabolic ketoacidosis and is accompanied by hyperglycemia. Small amounts of ketone bodies in the urine may occur during weight loss or starvation and pregnancy. Ketonuria may also indicate tubular dysfunction, when there is insufficient resorption of ketone bodies.
The main representative of ketone bodies in urine is β-hydroxybutyric acid, which makes up about 60–70%, acetoacetic acid is present in about 30–35% and acetone contributes only a few percent.
The detection of ketone bodies is based on the reactions of acetoacetic acid and acetone with sodium nitroprusside in an alkaline medium, which forms a red-violet-colored complex.[13] This principle is used by Legal's and Lestradet's tests, as well as diagnostic strips. Β-hydroxybutyric acid (ie the most abundant ketone substance) does not provide a reaction and therefore a negative result does not completely rule out ketoacidosis.[14]
Compounds with free sulfhydryl groups (the antihypertensive captopril or uroprotectant used in some mesna chemotherapeutic regimens) provide false positivity in urine ketone bodies[15]. Quite often, bacterial products in urinary tract infections also provide a similar response.
False negatives, apart from the already mentioned insensitivity of the β-hydroxybutyric acid tests, are not significant.
Other laboratory examination for patients with diabetes mellitus[edit | edit source]
Insulin (due to the method of the determination named also imunoreactive insulin, IRI). Because of large fluctuations in its levels throughout the day, interference with possible autoantibodies and the inability to distinguish endogenous insulin from medically administered one, the insulin is usually determined during exercise tests (oGTT). The values are reduced in type 1 diabetics, while they are usually increased in insulin resistance syndrome.
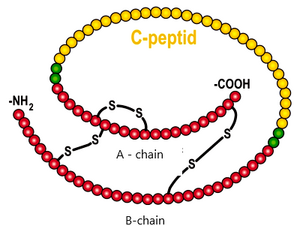
C-peptide is the part of the proinsulin molecule that is cleaved before an insulin secretion. Its serum concentration corresponds to the insulin secretion. It is not taken up by the liver and, as there is no C-peptide in the injected insulin, the results are not affected by the treatment. It crosses the glomerular membrane and therefore its concentration increases in renal insufficiency.
Type 1 diabetes is also associated with autoantibody positivity against Langerhans islets (ICA), insulin (IAA) and especially against one isoform of glutamate decarboxylase (GAD 654).
References[edit | edit source]
Related Articles[edit | edit source]
- Glucose in blood
- Diabetes mellitus
- Type 1 Diabetes mellitus
- Type 2 Diabetes mellitus
- Gestational diabetes
- Borderline disorders of glycoregulation
- Glycemia/determination
- Oral glucose tolerance test
- Glycated proteins
- Glucose in urine
- Ketone bodies in urine
- C-peptide
References[edit | edit source]
- ↑ ČEŠKA, Richard. Interna. 3. edition. Praha : Triton, 2020. vol. 970. ISBN 978-80-7553-780-5.
- ↑ ČEŠKA, Richard. Interna. 3. edition. Praha : Triton, 2020. vol. 970. ISBN 978-80-7553-780-5.
- ↑ NAIK, Ramachandra G – BROOKS-WORRELL, Barbara M – PALMER, Jerry P. Latent autoimmune diabetes in adults. The Journal of Clinical Endocrinology & Metabolism [online]. 2009, vol. 94, no. 12, p. 4635-44, Available from <https://academic.oup.com/jcem/article/94/12/4635/2596267>. ISSN 0021-972X (print), 1945-7197.
- ↑ Česká gynekologická a porodnická společnost. http://www.gynultrazvuk.cz/data/clanky/6/dokumenty/2019-05-gestastacni-diabetes-mellitus-dp-cgps-cls-jep-revize [online]. ©2019. [cit. 21-12-29]. <http://www.gynultrazvuk.cz/data/clanky/6/dokumenty/2019-05-gestastacni-diabetes-mellitus-dp-cgps-cls-jep-revize.pdf>.
- ↑ JOSEPH, Wang. Electrochemical glucose biosensors. Chemical reviews [online]. 2008, vol. 108, no. 2, p. 814-825, Available from <https://pubs.acs.org/action/cookieAbsent>. ISSN 0009-2665.
- ↑ HELLER, Adam – FELDMAN, Ben. Electrochemical glucose sensors and their applications in diabetes management. Chemical reviews [online]. 2008, vol. 108, no. 7, p. 2482-2505, Available from <https://pubs.acs.org/action/cookieAbsent>. ISSN 0009-2665.
- ↑ 1. Diabetes mellitus - laboratorní diagnostika a sledování stavu pacientů. 2015. Available from <http://www.cskb.cz/res/file/doporuceni/DM/DM_dop_201601.pdf>.
- ↑ 1. Diabetes mellitus - laboratorní diagnostika a sledování stavu pacientů. 2015. Available from <http://www.cskb.cz/res/file/doporuceni/DM/DM_dop_201601.pdf>.
- ↑ KAREN, Igor – SVAČINA, Štěpán. Diabetes mellitus : doporučené diagnostické a terapeutické postupy pro všeobecné praktické lékaře 2018. - edition. Společnost všeobecného lékařství ČLS JEP, 2018. ISBN 9788086998992.
- ↑ 1. Doporučený postup. 2016. Available from <http://www.diab.cz/dokumenty/doporuceni_DM_2015-2.pdf>.
- ↑ 1. Doporučený postup. 2015. Available from <http://www.cskb.cz/res/file/doporuceni/DM/DM_dop_201601.pdf>.
- ↑ MURRAY, Robert K – GRANNER, Daryl K – RODWELL, Victor W. Harper's Illustrated Biochemistry. 27. edition. New York : Lange Medical Books/McGraw-Hill, 2006. 190-194 pp. vol. 692. ISBN 978-0-07-125300-0.
- ↑ HOHENBERGER, E.F. – KIMLING, H. Compendium urinanalysis. Urinanalysis with test strip [online] . 1.. edition. Mannheim : Roche Diagnostics, 2008. vol. 105. Available from <http://www.diavant.info/diavant/servlet/MDBOutput?fileId=1392>.
- ↑ PLIVA-Lachema Diagnostika. PHAN® diagnostické proužky k vyšetření moče [online]. The last revision 2009-12-04, [cit. 2010-03-21]. <https://www.erbalachema.com/cz/>.
- ↑ HOHENBERGER, E.F. – KIMLING, H. Compendium urinanalysis. Urinanalysis with test strip [online] . 1. edition. Mannheim : Roche Diagnostics, 2008. vol. 105. Available from <http://www.diavant.info/diavant/servlet/MDBOutput?fileId=1392>.