Aging of the organism
By aging we mean the decline of vitality with age and the rise of susceptibility to various diseases. It is a universal event that looks the same in all organisms, but takes place at different speeds. From a molecular point of view, it is the inability to restore the correct structure of biomolecules indefinitely = "systemic molecular disorder" (Hayflick).
It is important to distinguish between two basic concepts:
- average life expectancy = statistical quantity; all members of the population in place and time (eg: year 2000 - men 71 years; women 78 years);
- maximum life expectancy = derived from how long the longest living people live; how long it is possible to live under optimal conditions (115-120 years); it does not change.
Pathogenesis of Aging[edit | edit source]
There are many theories regarding the aging process. It is generally accepted that with increasing age, errors accumulate in the body, which is unable to repair them sufficiently. Mitochondrial changes and the influence of radicals have the most significant influence.
Radical/mitochondrial theory of aging[edit | edit source]
As early as 1956, the theory of the accumulation of oxidative damage with age was created. Later, the mitochondrial theory was formulated: saying that mitochondria are the main source of oxygen radicals in the organism. Mitochondrial DNA mutates 10 times faster than nuclear DNA. mtDNA is not enveloped by histones and has a less perfect repair system. The formation of radicals in the mitochondria leads to the accumulation of mutations in the mtDNA. As a result, fce of respiratory complexes are disturbed → formation of radicals => heart failure, muscle weakness, DM, dementia, neurodegeneration. Mildly damaged mitochondria produce less energy than the cell needs.
Life-time Energy Potential[edit | edit source]
It is considered a proof of the mitochondrial theory. In most mammals, lifespan is determined by a certain sum of heartbeats/oxygen consumption. Smaller mammals have a more intense metabolism and a faster heartbeat, and therefore live shorter lives.
Theory of the accumulation of defective components in the cell[edit | edit source]
The second theory of aging processes is associated with the catabolic failure of the organism. There is an accumulation of defective components in the cell. Under normal circumstances, substances are broken down in several ways:
- short half-life proteins: proteasomes;
- long half-life proteins and organelles: autophagy (macroautophagy - whole organelles; microautophagy - macromolecules, small organelles; chaperones mediated autophagy);
- mitochondria: lysosomes.
If there is incomplete degradation in the lysosomes, iron is released from the mitochondria. Free oxygen radicals, lipoperoxidation, aggregation and polymerization of oxidized proteins and lipids are formed. lipofuscin (referred to as the pigment of aging) and defective mitochondria and protein aggregates are produced. They can initiate apoptosis.
The only way to get rid of waste material becomes cell division. The waste is not removed, but only divided into daughter cells, thereby reducing its concentration. The problem arises with cells that live a very long time and divide less: cardiomyocytes, hepatocytes.
Physiology of Aging[edit | edit source]
Various systems of the human body are affected during aging. There are changes in the nervous system (myelination of axons, number of synapses), locomotor apparatus, blood vessels and lungs are also affected. In people over 65, heart disease is one of the most common problems.
Basic Concepts[edit | edit source]
The maximum number of divisions a cell can go through before dying varies among cell types. It applies to all somatic cells, but not to tumor cells. In the cells of old people, the number of divisions is less.
Example: fibroblasts and epithelial cells never reach the Hayflick limit (they divide at most 50-70 times - a person does not live long enough for that many cell divisions to occur).
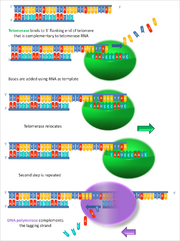
A ribonucleoprotein with its own RNA primer that complements the ends of chromosomes during DNA replication. Most cells in the human body do not need telomerase (they divide little or not at all). Stem, terminal and activated immune cells have telomerase. Telomerase is related to carcinogenesis.
Example: mouse somatic cells, unlike humans, have active telomerase. Experimental Knock-Out of Mouse Telomerase Gene Leads to Premature Aging.
How to slow down aging?[edit | edit source]
Antioxidants[edit | edit source]
They suppress the formation of free radicals, which are the cause of the disease state and have significant effects on pathogenesis. These are reducing agents capable of stopping radical chain reactions.
Examples: vitamin E (tocopherol), vitamin C (ascorbate), β-carotene, selenium (present in the active center of thioredoxin reductase and glutathione peroxidase - enzymes involved in antioxidant protection ).
However, antioxidant food supplements can also be harmful (carotene is among teratogens, vitamins E and A increase mortality). Vitamin C and selenium have no effect. Administration makes sense if the metabolism itself is defective!
Why they sometimes don't help:
- They do nothing in higher doses.
- Acts where they don't have: inhibition of the stress response, prevents the fight against infection, tumor cells, justified apoptosis.
- They also have effects other than antioxidant: tocopherols are anti-inflammatory, β-carotene is a co-carcinogen (with smoking or environmental toxins).
Caloric restriction[edit | edit source]
Limiting the amount of food while maintaining biological quality. It extends the maximum life span, reduces oxidative stress, the occurrence of tumors and slows down aging. An organism waiting for an unfavorable period (reduced food intake) devotes more energy to maintenance (less to reproduction).
Example: it also works in warm-blooded organisms (mouse) with a constant intensity of metabolism (we limit the amount to a quarter, it will extend the mouse's life up to twice)
Mechanism:
- suppression of IGF-I (somatomedin C) and insulin signaling;
- sirtuins = histone deacetylases, p53, etc.; inhibited by NADH, activated by NAD+.
Adequate physical activity[edit | edit source]
The need for energy stimulates the biogenesis and renewal of muscle mitochondria. An adequate dose of stress (movement) increases resistance to another → mechanism: induction of expression of heat shock proteins (chaperones) – stress response.
Example: production of ROS in muscle tissue during physical activity - beneficial (the body needs to renew mitochondria to replace damaged ones).[1].
Diet[edit | edit source]
A diet rich in fruit and vegetables is associated with a lower risk of cardiovascular disease, DM and some types of cancer (lung, mouth/pharynx) - but we don't know why (optimally 5x 80g per day).
Links[edit | edit source]
Related articles[edit | edit source]
References[edit | edit source]
- ↑ PLÁTENÍK, Jan. Aging [lecture for subject Pathobiochemistry 3, specialization general medicine, the 1st Faculty of medicine Charles University]. Prague. 1.12.2015. Avaliable from <https://ulbld.lf1.cuni.cz/prednasky-ke-stazeni>.
Used literature[edit | edit source]
- PLÁTENÍK, Jan. Aging [lecture for subject Pathobiochemistry 3, specialization general medicine, the 1st Faculty of medicine Charles University]. Prague. 1.12.2015. Avaliable from <https://ulbld.lf1.cuni.cz/prednasky-ke-stazeni>.