9.1 Physical Quantities & Units
In physical units we distinguish:
- basic unit;
- derived units;
- multiple and submultiple units;
- sub-units.
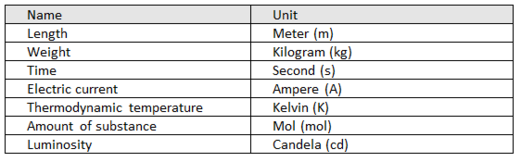
Basic quantities and their units
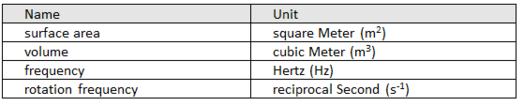
Derived quantities and units
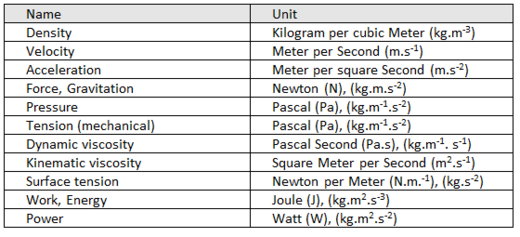
Derived units of MECHANICAL quantities
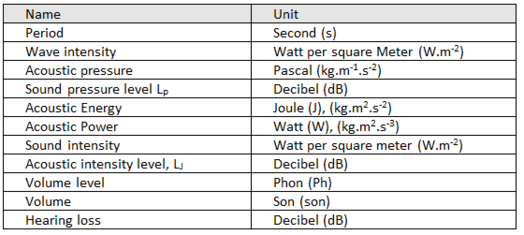
Derived units of ACOUSTIC quantities
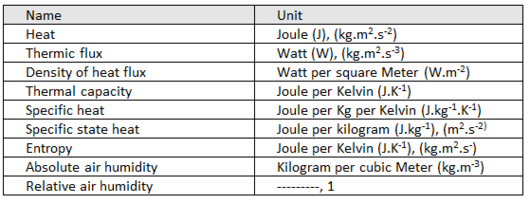
Derived units of THERMAL quantities
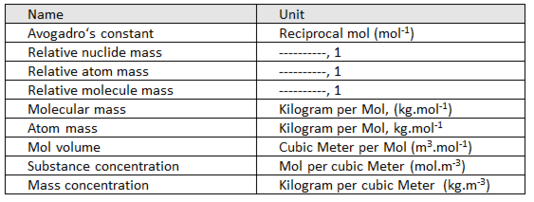
Derived units of MOLECULAR quantities
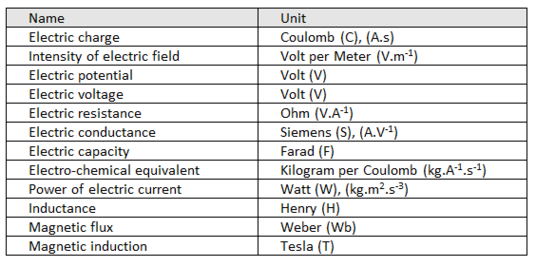
Derived units of ELECTRIC quantities
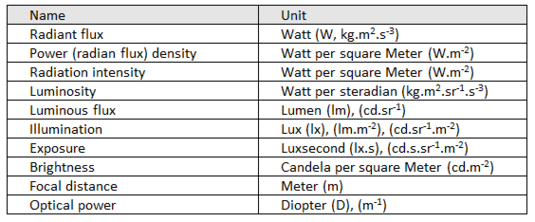
Derived units of OPTIC quantities
Derived units of quantities in ATOMIC PHYSICS and RADIOLOGY
9.1.1 Definition of quantities and units in RADIOLOGY
- Activity
The basic variable in radiochemistry is activity. Activity A, body of a radioactive element (isotope), is defined as the ratio of the differential radioactive transformations of N and time t
The main unit is: 1 becquerel = 1 Bq = 1 reciprocal second = 1 s-1.
Former unit is: 1 curie = 1 Ci = 3.7 * 1010 Bq.
- Half-life (of conversion) (T1/2)
Half-life (half-life of conversion) T1/2 mean median length of time for which any initial quantity of homogeneous atoms (nuclides) radioactive decay spontaneously just half of this:
T1/2 is the mean median length of time which any initial quantity of homogeneous atoms (nuclides) take to radioactively spontaneously decay to just half of their initial volume/amount/quantity:
(where) λ denotes the radioactive decay constant of the reference sample.
- Decay constant (λ)
Decay constant λ is understood as the variable characterizing the temporal instability of the radioactive element (isotope). It is the constant of proportionality between time (t) decrease in the number of atoms (N) radionuclide -dN/dt caused by spontaneous transformation, and the total number N of atoms of the same nuclide, which are still radioactively nondecayed. This begins with the time of the act of spontaneous radioactive decay and is defined as
Time-dimension is 1, then the reciprocal of the second unit, ie s-1.
Decay constant is a variable specific activity of the element relative to the total number N of nuclides in the sample. It is therefore also intended by the following formula:
Relationship with a median life indicates the relationship
Even in this context, recall the statistical validity of the law of decay
where N0 is the number of atoms in the initial nondecayed time tracking, and N nondecayed loss after time t. Decay constant for the unit Becquerel (Bq) is not used, is intended solely activity and units derived from it.
- Current density of particles
Particle current density J is the mean proportion of N particles that pass for some time t An a flat surface (perpendicular to the direction of motion of particles) and the size of the area and the time interval. In vector notation:
Unit is the reciprocal square meter per second, ie m-2s-1.
- Linear attenuation coefficient
Linear attenuation coefficient μ is the mean rate of decline in current density
J particle depends on the length of x in its path to the substance according to the relations:
The main unit of the linear attenuation coefficient is reciprocal meter, ie, m-1.
The inversion of the linear attenuation coefficient (1 / μ) thickness means a substance in which the current density particles are attenuated from the initial value to the value J0 = J / e ≈ 0.368 J0.
- Half-thickness
Half-thickness d1/2 is the mean layer thickness reducing the current density J of particle beam to half its original value. For the exponential absorption
where μ is the linear attenuation coefficient. The layer thickness is the same in the direction towards the current density particles.
- Mean lifetime
Mean lifetime of radioactive element τ means the average time necessary for the decreasing of the number N0 of atoms or nuclei of a species, which exist in a particular state to a number equivalent to the value of:
N = N0 / e
where e is the base of natural logarithms (e ≈ 2.718282). Size is 1 s.
- The total flux of particles
Total particle flux (emission sources) Φp is the ratio of the number ΔN of particles emitted by a source and time t in which the particles passed through the selected area of a certain size.
Dimension is time-1, the main unit is s-1.
- Shutter speed
Shutter speed (exposure power, before irradiation also speeds) mean rate of change of X irradiation
where ΔX denotes the mean irradiance increasing in the time interval t. The main unit of exposure rate is ampere per kilogram, A .kg-1.