Lipid bilayer
HOW ABOUT SOME BIOPHYSICS OF THE LIPID BILAYER? DID YOU GOOGLE IT?
|
Check of this article is requested. Suggested reviewer: Carmeljcaruana |
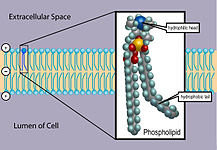
The lipid bilayer has been firmly established as the universal basis for cell-membrane structure. It is easily seen by electron microscopy, Each cell in the human body is composed of a unit membrane which separates the cytoplasm from the extracellular environment. The most important components of this membrane are phospholipids, cholesterol, proteins and chains of olgiosaccharides (glycolipids and glycoproteins). The plasmalemma ranges from 7.5 to 10 nm in thickness and appears as a trilamellar structure in electron microscopes after fixation in osmium tetroxide. Because all membranes of cells and cell organelles have this appearance, the 3-layered structure was designated the unit membrane, which is a phospholipid bilayer.
Molecular structure of a phospholipid[edit | edit source]
The most common phospholipid is lecithin (phosphatidyl choline) that consists of a non-polar (water-repelling) tail build by two long-chain fatty acids linked to a charged polar (water-attracting) head. Within the hydrophilic portion the molecule of glycerol is esterified with two fatty acid molecules (hydrophobic portion) and with one phosphate molecule which is again esterified with the aminoalcohol choline. The hydrophobic portion is made up by one saturated fatty acid (palmitic acid) and one unsaturated fatty acid (oleic acid). It is the shape and amphipathic (polar and non-polar) nature of the lipid molecules that cause them to form bilayers spontaneously in aqueous environments.
Arrangements of phospholipids in water[edit | edit source]
Monolayer[edit | edit source]
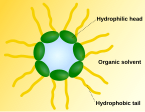
Phospholipid monolayers can be arranged either as a single surface film (e.g. in air and water or air and oil) or as micelles. A typical micelle is
spherical and forms an aggregate with the hydrophilic heads in contact with water, sequestering the hydrophobic single-tail regions in the micelle
centre. This phase is caused by the packing behavior of single-tail lipids in a bilayer. Micelles are important in fat metabolism.[1]
Bilayer[edit | edit source]
The hydrophilic / lipophobic polar heads of the phospholipids are directed toward the surface of the membrane, in direct contact with water. The hydrophobic / lipophilic nonpolar fatty acid chains are faced toward each other, away from water. Between the phospholipids van der Waals forces dominate which are relatively weak interactions between molecules based on diploes and intermolecular forces.
A special form of a bilayer can be found within liposomes or vesicles where it is arranged spherically. The liposome can be used as a vehicle for administration of nutrients and pharmaceutical drugs. it is composed of phosphatidylcholine-enriched phospholipids and may also contain mixed lipid chains with surfactant properties such as egg phosphatidylethanolamine. A liposome design may employ surface ligands for attaching to unhealthy tissue. The major types of liposomes are the multilamellar vesicle (MLV), the small unilamellar liposome vesicle (SUV), the large unilamellar vesicle (LUV), and the cochleate vesicle. Liposomes should not be confused with micelles and reverse micelles composed of monolayers.[2]
The bilayer structure is attributable to the special properties of the lipid molecules, which cause them to assemble spontaneously into bilayers even under simple artificial conditions.
Lipid composition and other components[edit | edit source]
The lipid composition of each half of the bilayer is different. In the outer leaf of the membrane phosphatidylcholie, sphingomyelins and many molecules of cholesterol can be abundant (espacially in red blood cells), whereas phosphatidylserine, phosphidylethanolamine and cephalin can be more concentrated in the inner half.
Cholesterol[edit | edit source]
Cholesterol molecules are interspersed throughout the lipid bilayer often at a ratio of 1:1, affecting packing and fluidity of fatty acid chains in means of restricting their movements. As a sterol (or modified steroid) and as a lipid molecule it is biosynthesized by many animal cells. Thus it is an essential structural component of cell membranes that is required to maintain both membrane structural integrity and fluidity.
Glycolipids and glycoproteins[edit | edit source]
The membrane not only contains lipids (phospholipids and cholesterol) but also proteins that are immersed in the bilayer. Both lipids and proteins may have externally exposed olgiosaccharide chains and are then designated as glycolipids and glycoproteins leading to asymmetry of membranes. This so-called glycocalix can be 20 nm thick and is species-specific as well as cell-specific, thus facilitating cell-cell recognition and substance uptake.
The A, B, O blood system is based on the fact that red blood cells have different carbohydrate chains on their cell surface. The presence of those specific antigens on the membranes of erythrocytes are the so-called aggultinogenes. Agglutinogenes are inborn, induce the formation of antibodies (agglutinines in the blood plasma) and are literally responsible for the agglutination of erythrocytes in incorrect transfusion. Every human has glycolipids and glycoproteins which have a particular type of carbohydrate chain which signals type O. The A type erythrocytes also have glycolipids and glycoproteins which have an carbohydrate called N-Acetylgalactosamine added to the basic type O carbohydrate chain. People with type B show an added galactose. Those with type AB have some glycolipids and glycoproteins with N-Acetylgalactosamine added and other glycolipids and glycoproteins with galactose added.[3]
Fluidity, lateral diffusion and fluid mosaic model[edit | edit source]
The lipid bilayer is fluid-like, permitting movement of imbedded molecules (lipids, proteins) sideways within the membrane. A classic experiment by Frye and Edidin showed that fluorescently labeled transmembrane proteins could move from place to place on the cell membrane.
The lateral diffusion adds an almost fluid character to the membrane. This fluidity is influenced by several factors:
- The higher the surrounding temperature, the higher is the level of fluidity. Below a certain ambient temperature, the membrane exists in a vicous-crystalline form.
- The degree of saturation and the length of the fatty acids have an impact on the level of lateral diffusion. Long chains develop more van der Waals forces, the cohesion becomes more stabile and the fluifity decreases. Unsaturated fatty acids accumulate "kink" because of their cis-double bonds. Those irregularities lead to lesser cohesion, lesser van der Waal's interactions and to an increase of fluidity.
- Cholesterol acts as a "fluidity buffer" at higher and lower temperatures and prevents the collapse of the plasma membrane during thermical pressures. Cholesterol-rich domains in the bilayer are called lipid rafts.
The fluid mosaic model emphasizes the mosaic disposition of membrane proteins and the fluid nature of the lipid bilayer. Many proteins inserted in it or bound to the cytoplasmatic surface (peripheral proteins) move within the fluid lipid phase. Hydrophobic amino acids of integral membrane proteins interact with the hydrophobic fatty acid portions of the membrane. Some integral membrane proteins are not bound rigidly in place and are able to move within the plane of the cell membrane, but unlike lipids, most of the membrane proteins are restricted in their lateral diffusion by attachment to cytoskeletal components. In most epithelial cells, tight junctions (Zonula occludens) also restrict lateral diffusion of unattached transmembrane proteins and lipids.
Membrane splitting and cyrofracture[edit | edit source]
Membrane splitting occurs along the line of fatty acid tails of the phospholipids, because only weak hydrophobic interactions bind the halves of the membrane along this line. When cells are frozen and fractured (cyrofracture), the lipid bilayer is often cleaved along the hydrophobic center. Electron microscopy of cyrofrature preparation replicas is a useful method of studying membranous structures. Most of the protruding membrane particles seen are proteins that remain attached to half of membrane adjacent to cytoplasm (= P or protoplasmic face). Fewer particles are found attached to the outer leaf of membrane (= E or extracellular face). For every protein particle that bulges on one surface, a corresponding depression appears in the opposite surface.
References[edit | edit source]
Huss, Sebastian (2012): Biologie Band 1. MEDI-LEARN Skriptenreihe. 5. Aufl. p. 2-4.
Mescher, Antony (2010): Junqueira's Basic Histology. Text and Atlas. 12th edition. p. 18-21.
Notes (2014): Biophysics. prof. RNDr. Evžen Amler, CSc. 2nd faculty of medicine, Charles University, Prague. Czech Republic.
Paulsen, Douglas (2010): Histology & Cell Biology. 5th edition. p. 26-27.
- ↑ http://en.wikipedia.org/wiki/Micelle
- ↑ http://en.wikipedia.org/wiki/Liposome
- ↑ http://www.ncbi.nlm.nih.gov/books/NBK21744/