Hemoglobinopathies
Template:Infobox - disease It is one of the most common hereditary diseases in the world. They are characterized on the one hand by a change in the formation of the entire globin chain or by the synthesis of an abnormal globin chain. We observe an increased incidence of thalassemia and sickle cell anemia in areas with malaria. Indeed, heterozygous forms provide some protection against malarial infection.
Hemoglobin mutation[edit | edit source]
Mutations of the beta gene affect 50% of hemoglobin chains in heterozygotes; the alpha gene mutation affects only 25% of molecules, but they manifest themselves before birth. Hereditary diseases caused by impaired formation of hemoglobin (haemoglobinopathy) can be divided into:
- diseases with a disorder in the structure of the globin chain;
- causing hemolytic anemia – sickle cell anemia, unstable hemoglobins;
- reducing the ability of hemoglobin to carry oxygen - methemoglobinemia;
- diseases with a disorder of synthesis of the globin chain - thalassemia.
Hemoglobinopathies were the first hereditary diseases for which DNA diagnosis was successful.
Mechanism of hemoglobinopathies:
- About 250 different structural mutations of hemoglobin have been described - ⅔ of them affect the β-chain, less than ⅓ the α-chain.
- Substitution of one base – change of AMK in the structure of hemoglobin (HbS).
- Two substitutions in different codons - changes two AMK (HbC Harlem).
- The length of the hemoglobin chain can be shortened by deletion or, conversely, lengthened by mutation of the stop codon.
- Unequal crossing-over – ex Hb-Lepore – non-α-chain was formed by recombination of the first part of the DNA delta-chain and the second part of the β-chain.
Diseases with a change in the structure of the globin chain[edit | edit source]
Disorders of hemoglobin structure causing hemolytic anemia[edit | edit source]
Sickle cell anemia - this is a severe autosomal recessive hereditary hemolytic anemia, associated with impaired growth, spleen dysfunction and so-called vaso-occlusive crises - these are caused by clogging of erythrocyte capillaries in the limbs, spleen and lungs. Without proper medical care, the disease is fatal. Heterozygotes are clinically healthy (or have very little clinical manifestations), only part of the erythrocytes show sickling.
The mutation is caused by the substitution of AMK in the polypeptide in the β-globin chain (point mutation). Valine is included in the sixth position instead of acid. glutamic. We refer to the changed hemoglobin as HbS. The reason for the confusion is the change of one nucleotide (A–T) in the GAG triplet, which causes a change in the isoelectric point of hemoglobin (instead of negatively charged glutamic acid, there is non-polar valine). When the partial pressure of oxygen is reduced (i.e. when deoxyhemoglobin is formed), HbS molecules aggregate into rod-like polymers, reducing the plasticity and increasing the fragility of erythrocytes. Sickle-shaped erythrocytes have a harder time passing through the capillaries, they can clog them and cause local hypoxia and infarction. During passage through the lungs and oxygenation, the shape is adjusted again, but after some time the pathological sickle-shaped shape is fixed (irreversibly sickle-shaped erythrocytes)[1].
Consequences:
- blood stagnation – hypoxia;
- aggregation of blood cells in blood vessels – ischemia and heart attacks;
- increased destruction of blood cells in the spleen - anemia.
Clinical picture:
- hemolytic anemia with an increased occurrence of reticulocytes, jaundice and a tendency to develop cholelithiasis;
- reduced dysfunctional spleen (ischemia, heart attacks and infections);
- various "symptoms" (acute and chronic) "resulting from ischemia" (neurological symptoms - motor and sensory disorders; visual disturbances, pulmonary microinfarcts - chest pain, pulmonary hypertension, muscle pain, aseptic femoral head necrosis, papillary kidney necrosis with hematuria, skin ulceration...);
- crisis refers to sudden attacks of strong, long-lasting bone and muscle pain, which are often caused by a stay at high altitude, impaired lung function, increased metabolic activity and dehydration.
Therapy:
Early diagnosis is needed for effective treatment and minimization of complications. Newborn screening is therefore introduced in some countries of the Middle East and the USA.
- Antibiotic prophylaxis (greater vulnerability to infections arising from spleen dysfunction).
- Blood transfusion.
- Transplantation of hematopoietic stem cells (bone marrow).
- Pharmacologically, hydroxycarbamide (hydroxyurea) - increases the gene expression of HbF (fetal hemoglobin - α2γ2), which does not contain defective β-subunits.
- Gene therapy - was described (2017) using a lentiviral vector (however, its widespread implementation in the areas of the main incidence in developing countries is unrealistic for financial reasons).
This disease is rare in our country, but the HbS allele has a high frequency in many areas of the world - the Mediterranean, Arabia, India and especially sub-Saharan Africa (Nigeria, the Democratic Republic of the Congo and Tanzania); affects the black population in the USA (up to 8% frequency)[1] In malarial areas, it is possible to observe positive selection against heterozygotes - they are more resistant to this protozoan infection. This is probably due to the reduced ability of Plasmodia to reproduce inside the sickle-shaped erythrocytes, as well as an increase in the total number of red blood cells.
Note From a genetic point of view, there is an interesting co-inheritance with polymorphisms in genes related to HbF gene expression (HGB2, BCL11A, ...) as a result of which the HbF level is higher (up to 10%). These polymorphisms are also positively selected. Hemoglobin C (HbC) – at the 6th position in the β-chain instead of glutamic acid lysine. The mutation is conditioned by the substitution of guanine for adenine (GAG – AAG). HbC is less soluble and crystallizes in erythrocytes; the symptoms of hemolytic anemia are the same as sickle cell anemia.
Hemoglobinopathies affecting the ability of hemoglobin to carry oxygen[edit | edit source]
These mutations are an example of a mechanism of change in protein function. The stability and synthesis of hemoglobin are not affected by these mutations.
Methemoglobins (HbM) – the mutation affects the binding site of globin and heme, the so-called heme pocket. The action of reductases on the binding of iron and oxygen is thus affected (the mutation leads to a change in the oxidation number of Fe2+ to Fe3+, which does not bind oxygen). Physiologically, HbM is produced to a small extent, but is reduced by methemoglobin reductase. In HbM Hyde Park, AMK Histidine is replaced by Tyrosine at position 92 in the β-globin chain. The consequence is a tighter binding of oxygen to iron and a slow release of oxygen by reductase in the tissues. Heterozygotes are cyanotic but without signs of hypoxia. Homozygotei are not known, the combination is apparently early lethal.
Short-term methemoglobinemia may occur in persons with HbA after nitrate poisoning; especially infants in which the reductase is not yet active are at risk.
Diseases with globin chain synthesis disorder - thalassemia[edit | edit source]
Thalassemias are the most common monogenically inherited diseases (AR). It is a heterogeneous group of diseases with a disorder in the synthesis of the α-globin chain (α-thalassemia) or β-globin chain (β-thalassemia). The second chain is synthesized in normal amounts and, being in relative excess, precipitates in erythrocytes and causes their premature destruction and aggravates hypochromic anemia. Thalassemia heterozygotes are more resistant to malaria.
Thalassemia:
Thalassemia 2:
α-Thalassemia[edit | edit source]
The disease is caused by a disorder in the formation of the α-globin chain, the formation of both fetal and adult hemoglobin is damaged. Instead of HbA, hemoglobin is formed from four gamma chains or four beta chains - these tetramers are unable to carry oxygen. Anemia is microcytic, hypochromic, with extravascular hemolysis and splenomegaly, in severe cases the fetus suffers from a lack of oxygen, it develops hydrops and other damage.
Deletions are the most common cause; the presence of two identical α genes on the 16th chromosomeu increases the probability of an unequal synapse; subsequent crossing-over results in a triplication of the α gene on one chromosome and a deletion on the other. There are two genes (four alleles) for the α-globin chain in the genotype, the severity of the disease corresponds to the number of affected alleles:
- deletion of one allele is without clinical symptoms;
- deletion of two causes thalassemia minor (perhaps trans form A−/A−, or cis form −−/AA) and mild anemia;
- deletion 3 allele – severe hemolytic anemia with the formation of unstable hemoglobin HbH (β4), which precipitates on the membrane and cytoskeleton of erythrocytes as Heinz bodies;
- deletion of four alleles lethal already during intrauterine development, Bart's hemoglobin (γ4) or HbH (β4) can be formed.
β-Thalassemia[edit | edit source]
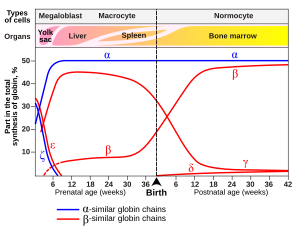
β-thalassemia also causes anemia, but only after the 3rd month of life, when the synthesis of HbF (γ-chain) is replaced by the synthesis of HbA (β-chain). An excess of α-chains damages erythroid cells and they disintegrate already in the bone marrow (ineffective erythropoiesis), with a complete absence of β-chains, HbF (α2γ2) and HbA2 (α2δ2). There is an increased amount of HbF and HbA2 in erythrocytes, as the production of γ and δ chains is not affected.
The β-chain may be insufficiently synthesized (β+ thalassemia) or not produced at all (β0 thalassemia).The β-chain is encoded by 5 genes on the 11th chromosome.
β-thalassemia can be caused by all known disorders of the proteosynthesis mechanism: promoter mutation (mild BT, Japan), deletion of part of the β-globin gene (severe BT, Indians), chain fusion (severe BT Lepore, Italy), splice site mutation mRNA and the creation of a new splice site (severe BT, Africa), cap site mutation (mild BT, Asia), disruption of polyadenylation of the mRNA end (mild BT, Africa), formation of a stop codon (severe BT, Mediterranean) or reading frame shift (severe BT, Indians).
Anemia is microcytic, hypochromic, pronounced hepato- and splenomegaly, bone marrow hyperplasia. In the treatment, repeated transfusions of erymase are necessary (not only to replenish hemoglobin, but also to reduce the stimulation of erythropoiesis), but there is an excess of iron - the formation of oxygen radicals damaging various organs (liver - cirrhosis, pancreas - DM, myocardium - fibrotic changes).
Links[edit | edit source]
Related Articles[edit | edit source]
- Structure of Hemoglobin
- Hemoglobin and its derivatives
- Anemia
- Hemolytic anemia corpuscular
- Hemolytic anemia extracorpuscular
External links[edit | edit source]
References[edit | edit source]
Source[edit | edit source]
- ŠTEFÁNEK, Jiří. Medicína, nemoci, studium na 1. LF UK [online]. [cit. 2010-02-11]. <http://www.stefajir.cz>.
- PASTOR, Jan. Langenbeck's medical web page [online]. [cit. 2010-04-12]. <http://langenbeck.webs.com>.
- NEČAS, Emanuel. Patologická fyziologie orgánových systémů : Část I. 2. edition. V Praze : Karolinum, 2009. 379 pp. pp. 63. ISBN 978-80-246-1711-4.