Formation and detoxification of ammonia, urea cycle and its regulation, hyperammonaemia
Ammonia-NH3 synthesis[edit | edit source]
Ammonia-NH3 is a toxic weak basic compound that needs to be detoxified and eliminated from the body. Ammonia derives from the metabolism of amino acids and especially that of gluconeogenesic transversion of amino acid into glucose. Glucose can be synthesized from amino acid carbon skeleton after the removal of its amino group. This removal of the amino group occurs through two similar processes, transamination and deamination.
- Transamination: it is a process of transferring amino groups from one molecule to another. There is no formation and no excretion of ammonia, thusly no net change in the nitrogen amount of body. It is a process involved in amino acids in which the amino group is transferred from the amino acid to a certain α-ketoacid with the consequent formation of a second α-ketoacid and amino acid. The reaction is catalysed by the enzyme aminotranferase (aka transaminase) which requires pyridoxal phosphate as a prosthetic group. All transaminases contain this prosthetic group which derives from pyridoxine a water soluble vitamin also known as vitamin B6. The amino group from amino acids is temporarily uptaken by the pyridoxal phosphate as pyridoxamine phosphate prior to its donation to an α-ketoacid. All amino acids except lysine, threonine, proline and hydroxyproline participate in transamination process.
- Deamination: it is a process of removing amino groups from one molecule in order to reduce the amount of nitrogen of the body through ammonia synthesis and elimination. It is a process occurring in the liver during the metabolism of amino acids. The amino group is removed from the amino acid and converted to ammonia-NH3 whose toxic activity is cancelled by conversion into urea which is eventually excreted. The glutamate dehydrogenase-GDH enzyme,present in liver, occupies a central role in nitrogen metabolism. Glutamate amino acid is cleaved into α-ketoglutarate and ammonia a reaction catalysed by GDH in a process called deamination. Glutamate is the only amino acid that undergoes oxidative deamination at a relatively high rate. The formation of ammonia from the amino group thusly occurs mainly via the amino group of glutamate. Soham Ganguly (talk) 19:56, 3 August 2015 (CEST)
Urea cycle[edit | edit source]
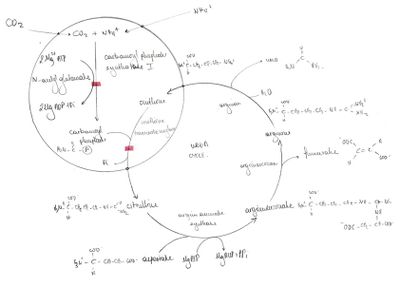
Ammonia detoxification[edit | edit source]
Ammonia is rapidly removed from the circulation in the liver, converted into a water soluble compound known as urea. Ammonia is toxic to the CNS because it reacts with the α-ketoglutarate to form glutamate. As a consequence, the depleted levels of α-ketoglutarate impairs the function of the Citric Acid cycle in neurons, depriving them energy production. Furthermore, glutamate is a potent CNS neurotransmitter, thus any significant increase in the concentration of glutamate could have abnormal effects in synaptic transmission.
| Step | Reactants | Products | Catalyzed by | Location |
|---|---|---|---|---|
| 1 | NH4+ + HCO3− + 2ATP | Carbamoyl phosphate + 2ADP + Pi | Carbamoyl phosphate synthetase I | mitochondria |
| 2 | Carbamoyl phosphate + Ornithine | Citrulline + Pi | Ornithine transcarbamoylase | mitochondria |
| 3 | Citrulline + Aspartate + ATP | Argininosuccinate + AMP + pyrophosphate | Argininosuccinate synthetase | cytosol |
| 4 | Argininosuccinate | Arginine + Fumarate | Argininosuccinase | cytosol |
| 5 | Arg + H2O | Ornithine + Urea | Arginase | cytosol |
Urea cycle regulation[edit | edit source]
Urea cycle is regulated by the rate limiting enzyme carbamoyl phosphate synthase I, the first enzyme of the ammonia detoxification pathway. It is only active in presence of its allosteric activator N-methyl-glutamate amino acid. It catalyses the condensation of ammonium ions NH4+, CO2 and ATP to form carbamoyl phosphate, a product that will condense with L-ornithine in order to initiate the urea cycle.
Links[edit | edit source]
Bibliography[edit | edit source]
MURRAY, Robert K. – BENDER, David A.. Harper's Illustrated Biochemistry. 29th edition. McGraw-Hill Companies, Inc., 2012. ISBN 978-0-07-176576-3.