Fluorescence quenching
Introduction[edit | edit source]
Quenching of fluorescence is a physicochemical PROCESS that decreases fluorescent intensity of light emitting molecules. There are two different ways of quenching: static and dynamic quenching. It is to be mentioned that there is a difference between quenching and decrease of fluorescence due to the high state of molecular excitement or chemical changes of FLUOROPHORE (like oxidation).
Form of Quenching[edit | edit source]
Static Quenching[edit | edit source]
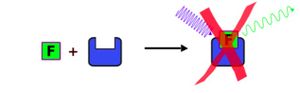
Static quenching (or contact quenching) of fluorescence is, when a Fluorphor (F) and a Quencher (Q) are creating a NON-FLUORESCENT complex (FQ) BEFORE EXCITATION OF F. The chemical equation therefore is: F + Q ⇌ FQ
The chemical equilibrium (Ks) between the Fluorphore, the Quencher and the complex FQ is formed by the law of mass action and equal to the Stern-Volmer constant (Ksv). There [FQ] stands for the concentration of the complex FQ, [F] for the concentration of the loose Fluorfore and [Q] for the loose Quencher.
The Stern-Volmer-equation describes the dependence of the fluorescent intensity of a fluorescent dye on the concentration of an quenching material (Quencher). It was created by the two physical chemists Otto Stern and Max Volmer in 1919. There F0 is the fluorescent intensity of the fluorescent dye without the quencher, F is the fluorescent intensity of fluorescent dye with the quencher. Ks stands for the Stern-Volmer-constant and [Q] for the concentration of the quencher.
Dynamic Quenching[edit | edit source]
Quenching method that occurs between two light-sensitive FLUOROPHORES (called donor and acceptor fluorophores). Exchange of ENERGY between donor and acceptor fluorophore happens AFTER EXCITATION – ACCEPTOR MAY EITHER QUENCH THE ENERGY OR RELEASE A LOWER ENERGY PHOTON. Dynamic quenching methods, explained further are: Förster Energy Transfer, Dexter Energy Transfer and Exciplex.
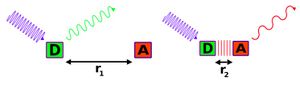
Förster Energy Transfer[edit | edit source]
Donor fluorophore transmits ENERGY to acceptor fluorophore without coming in direct contact. Fluorescence Resonance Energy Transfer (FRET) is a technique for measuring distance between two light sensitive molecules AS THE EXTENT OF THE PROCESS IS this process is so distance-dependent. The most useful application is measuring the size of large molecules. A donor-acceptor pair of fluorophores is attached to a big molecule and length is measured over the time that passes while donor transmits energy to the acceptor.
Application: Information is gathered only in case if no conformational changes occur to the subject molecule. If the shape or size of molecule is compromised, then information gathered will be useful for analyzing dynamical protein-based processes occurring between two fluorophores attached to the subject. Today, this technique is widely applied in many fields such as single-molecule experiments, molecular motors, biosensors and DNA mechanical movements.
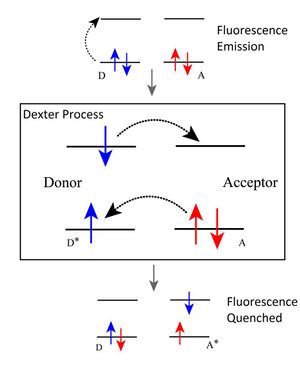
Dexter Energy Transfer[edit | edit source]
Similarly to Förster Energy Transfer, is a non-radioactive and short range process; though by principle and range they are different. Dexter Energy transfer happens only when two chemical groups (fluorophores- intermolecular) or two parts (fluorophores-intramolecular) of a chemical group collide and exchange ENERGY. Ground-State group (G) might come into contact with Excited, fluorescent group (E) and take away the ENERGY that accounts for fluorescence. Dexter energy transfer stands out as it requires a so-called “Wavefunction Overlap” between two fluorophores, in other terms; - atoms on molecules or chemical groups need to overlap their electron clouds. An energy transferring electron will travel from donor to acceptor and occupy acceptor’s least occupied orbital. Energy Transfer results in donor returning from excited to ground state and acceptor maintaining the ground state due to greater electronegativity it has. Now that the energy is transferred there are no fluorophores that are excited and emit light, and we can say that the quenching process has happened.
Application: Dexter energy transfer had been acclaimed as one of premier phenomena upon which photochemistry relied. Nowadays its application is more popular in emissions fields, in such as energy converters and organic LED’s
Exciplex[edit | edit source]
An Excimer is a very shortly existing molecule, either dimeric (consisting of two similar monomers A-A) or heterodimeric (consisting of two different monomers A-B). An Excimer cannot exist if both monomers are in ground-state phase. Therefore a dimer or a heterodimer has to consist of an inert group or element (with full octet; e.g. a noble gas) and an excited group or element. “Exciplex” (short for excited complex) is the other name for a heterodimeric excimer. Quenching process performed by Exciplexes relies heavily upon their “CT” (charge-transfer) character. An exciplex binds covalently to a target molecule, fluorescence of which needs to be quenched, and the resultant chemical entity is referred to as the “Charge Transfer Complex”. In The charge transfer complex itself, starts the quenching process.
A CT complex is also called “Electron-Donor-Acceptor complex”; this means that a CT complex may be created by two fluorophores and a molecule, or be present in one large molecule as its two different parts providing the charge transfer capability. Fluorophores usually ought to bind to a large fluorescent macromolecule in order to form a CT complex and start decreasing its fluorescence. Unpaired electrons on the ends of both, - fluorophores and the macromolecule form covalent bonds (spin-pairs) and the energy dissipated by covalent bonds tends to decrease the CT complex energy as well as its fluorescence. The Process causes CT complex to stabilize and therefore is often called “CT Stabilization”. In another scenario, where donor-acceptor functionality is present within a macromolecule itself, the simple electron transfer happens after fluorescence is given off at a point at which molecule can no longer maintain its stable conformation and “performs” CT in order to stabilize.
Application: This form of quenching and forming covalent bonds is of particular interest to fields of biology that rely on fluorescence spectroscopy techniques mainly as, and for most research means. There exists evidence indicating that CT stabilization leads to a conical intersection (concentration electrical energy in the center) of subject molecules of quenching, which is useful for analyzing electrical and dynamical activity data gathered during quenching and other chemical processes that a subject molecule undergoes.
Medical Applications[edit | edit source]
Quenching is a really good visible physical phenomenon, that's why it is used as an indicator for molecular processes.
- Indicator for DNA hybridization
If DNA strand is hybridizing with an opposite strand it gets quite linear and stiff. Fluorphore and Quencher are attached to the ends. If the bases are paring correctly the Fluorphore and Quencher disconnect and the quenching stops.
- Indicator for Potassium-ions
The ends of short DNA fragments (telomer sequence), connected with a fluorescent dye and quencher through a covalent bond, are divided in a solution. The dye is producing light. If the there are any potassium-ions present, the DNA fragment is wrapping around the potassium-ion and the endings are touching each other. The fluorescence is quenched (lightning stops).
Conclusion[edit | edit source]
Quenching as a physicochemical discipline proves to be irreplaceable as a means to laboratory tests research. Different types of quenching enable extracting information about molecules and dynamic and electrical processes occurring with them. Development of quenching mechanisms implies advances in gathering data and helping organic and inorganic chemistry in synthesizing drugs in fields of pharmacology and chemical engineering.Objective is increased efficiency and decreased recovery period from treatment. Further vision predicts better biochemical understanding of neural activity during normal and treatment periods of a patient. THIS CONCLUSION IS FULL OF IMPORTANT SOUNDING BUT MEANINGLESS WORDS - AVOID IN FUTURE
Sources[edit | edit source]
- http://webcache.googleusercontent.com/search?q=cache:nIBmzlF_TGIJ:www.jh-inst.cas.cz/~fluorescence/support/Lectures/UFCH_fluor04.pps+&cd=4&hl=de&ct=clnk&gl=cz&client=safari
- http://www.chemie.de/lexikon/Quenching-Effekt.html
- http://home.arcor.de/trenbolon/presentation_5.pdf
- http://www.chemie.de/lexikon/Stern-Volmer-Gleichung.html#Die_Stern-Volmer-Konstante_bei_statischer_Fluoreszenzl.C3.B6schung.html
- https://www.rose-hulman.edu/~brandt/Fluorescence/Quenching_processes.pdf
- http://dwb.unl.edu/Teacher/NSF/C08/C08Links/pps99.cryst.bbk.ac.uk/projects/gmocz/qch.htm
- http://chemwiki.ucdavis.edu/Theoretical_Chemistry/Fundamentals/Fluorescence_Resonance_Energy_Transfer
- http://chemwiki.ucdavis.edu/Theoretical_Chemistry/Fundamentals/Dexter_Energy_Transfer
- http://www.sciencedirect.com/science/article/pii/0009261474850785
- http://www.sciencedirect.com/science/article/pii/0022231388901809
- Prof. Dr. Spichiger-Keller, U. E. (Sept. 2008), Chemical Sensors and Biosensors for Medical and Biological Applications, John Wiley & Sons, ISBN: 9783527288557
- Lakowicz, J. R. (June 2010), Principles of Fluorescence Spectroscopy, 3rd, Springer, ISBN: 978-0-387-31278-1