Biological membrane
The biological membrane (biomembrane, cell membrane) is present on the surface of cells and forms a semipermeable barrier between two compartments (cellular contents and extracellular environment). It is also present on the surface of membrane organelles in the cell, such as mitochondria, Golgi apparatus or endoplasmic reticulum. It has many important functions, such as transporting substances, receiving information from its surroundings and also serving for mutual recognition and communication between cells.
Structure[edit | edit source]
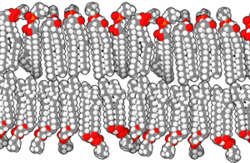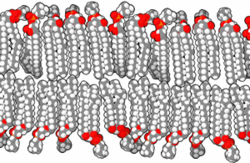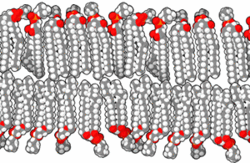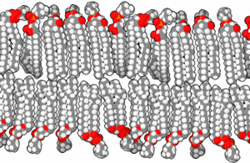
The basic building block of biological membranes is the lipid bilayer. Membrane proteins are embedded in the lipid bilayer, which give the membrane its specific functions and properties. The outer surface of membranes may contain carbohydrates that function as signalling molecules. Cholesterol molecules are also found in mammalian cell membranes. The biological membrane is about 5 nm thick.
Lipids[edit | edit source]
Lipids are substances of biological origin (chemically they are esters of alcohols and higher fatty acids). In biological membranes, lipids are mainly found with two hydrocarbon aliphatic chains, which usually contain other substances (e.g. a phosphoric acid residue or a carbohydrate). It is these molecules that cause the so-called amphiphilic character of lipids. While the alcohol molecule and the two carbon chains attached to it are hydrophobic, the phosphoric acid forms the hydrophilic part. This fact leads in aqueous environments to the formation of ordered structures (micelles, liposomes, lipid bilayers) that orient the lipids with their polar head towards the water and their hydrophobic tails inwards, thus protecting them from contact with water. The behaviour described is based on electrostatic interactions and on the formation of hydrogen bridges.
There are a variety of lipids in the human body, but they can be divided into two basic groups, glycerol phospholipids and sphingolipids. If it is a lipid with a phosphate head we generally speak of phospholipids.
Proteins[edit | edit source]
Only a few substances can pass freely through the lipid bilayer, the others must be transported across the membrane by proteins. Thus, proteins create specific properties of cell membranes and are also specifically distributed.
Proteins perform a variety of tasks in membranes. In addition to the aforementioned transfer of ions, metabolites and nutrients, proteins can also anchor the membrane to macromolecules inside or outside the cell. Many proteins also function as receptors to recognize chemical signals in the environment. This is very important for communication between cells. Proteins exhibiting enzymatic activity catalyze specific reactions. Special filamentous proteins then form the cell cortex, which forms a submembrane network attached to the membrane surface. This supports and reinforces the cell membranes and helps maintain the shape of the cells.
Integral proteins are tightly bound to the membrane by hydrophobic forces. It is very difficult to detach them from the membrane; they extend all the way between the lipid chains. Integral proteins, like phospholipids, are amphiphilic, the parts exposed to water are hydrophilic, and the parts classified as lipids are hydrophobic (non-polar). If such a protein passes through the entire lipid bilayer, we speak of penetrating/transmembrane proteins; if it passes through only part of the membrane, it is a non-penetrating protein.
Peripheral proteins are perched on the surface of membranes by electrostatic forces or by the formation of hydrogen bridges. They associate with integral proteins and are relatively easy to detach from the membrane.
Carbohydrates[edit | edit source]
The surface of the biological membrane of eukaryotic cells is often supplemented with carbohydrate molecules. These can attach to both lipid and protein molecules (so-called glycoproteins). All carbohydrates are found only on the outside of the membrane, where they form a sheath called the glycocalyx. This serves as a protection against damage and gives the cell a mucilaginous surface (both oligosaccharides and polysaccharides are able to absorb water). This is mainly used by motile cells such as red blood cells. Furthermore, carbohydrates serve as a recognition marker for cells, as they can form very diverse formations.
Membrane fluidity[edit | edit source]
In 1972, S. J. Singer and G. L. Nicolson developed a model of biological membrane structure, the so-called fluid mosaic model, which incorporates the observation that the lipid bilayer is a two-dimensional fluid in which the individual components are not rigidly bound in one place but can move around in different ways (although not completely freely).
The degree of membrane fluidity expresses how easily the lipid molecules move in the plane of the bilayer. It depends on the representation of the individual components and must be kept within certain limits. The degree of fluidity at a given temperature depends on the phospholipids, and also on the nature of the hydrocarbon chains . The more tightly and regularly the chain can pack, the more viscous and less fluid the bilayer will be. Two of the properties of hydrocarbon chains, length and saturation, in particular affect their arrangement. Shorter chains reduce the tendency of the hydrocarbon ends to interact with each other and therefore increase the fluidity of the bilayer. Each double bond creates a small irregularity that makes it difficult to attach one chain to the other. Lipid bilayers with more double bonds are more fluid. In animal cells, membrane fluidity is reduced by the presence of cholesterol, which fills the gaps between adjacent phospholipid molecules in the membrane. The bilayer is strengthened by cholesterol, reducing its fluidity and permeability. This is exploited by bacteria and yeasts, for example, which must adapt to changing temperature conditions. In their cells, chain lengths and composition are constantly changing to maintain the fluidity of the membrane.
The fluidity of the biomembrane is important to the cell for many reasons. It allows membrane proteins to interact and diffuse rapidly in the plane of the membrane, which is important, for example, in cell signalling. It allows membrane lipids and proteins to translocate from where they were incorporated after their synthesis to other sites in the cell. It allows membranes to fuse and mix their molecules. It also ensures that membrane molecules are evenly distributed among daughter cells during cell division.
Mobility of membrane components[edit | edit source]
The lipid bilayer retains an organized structure, but its individual molecular components perform random motions (Brownian motion) characteristic of the liquid state.
It has been shown that phospholipid molecules constantly perform rapid rotational and translational movements in the bilayer. The frequencies of these movements are in μs-¹ and the positions of two adjacent molecules are exchanged about 10⁷ times per second. Proteins vary considerably in their mobility in the biomembrane. Some of them move continuously like lipids, others, forming channels in the membrane, stand still.
Thus, membrane components perform several different modes of movement. Rotation of whole molecules within the membrane surface, lateral movement within the membrane, and flipping of molecules from one lipid layer to another are all possible.
Rotational motion (i.e., rotational diffusion) is described by the rotational diffusion coefficient as
,
where kB is the Boltzmann constant, r is the radius of the rotating molecule, h is its height, and η is the viscosity of the surrounding medium.
Another movement that takes place in the membrane is lateral diffusion - the so-called "floating" through the membrane sheet. In a membrane forming a two-dimensional liquid, the building lipid molecules move freely in their own layer in any direction in the plane of the membrane. The derivation of the lateral diffusion coefficient DL is based on Einstein's equation for Brownian motion and has the form
,
where d is the average distance between the molecules in the membrane and v is the frequency of the molecule skips. The distance x that a membrane molecule travels in time t can be determined using Einstein's equation for a two-dimensional system, where
.
The last, relatively rare type is flip-flop movements (transverse diffusion). In proteins they hardly occur at all. Another important feature of biomembranes is structural and functional asymmetry. It is manifested both in the distribution of proteins and in the different composition of the inner and outer lipid layers. The distribution of the different polar lipid types in the two membranes is arranged to minimize the frequency of flip-flop movements. Specific, ATP-dependent enzymes, called flippases, exist to flip lipids as they are inserted into the biomembranes.
Permeability of membranes[edit | edit source]
Transfer of non-polar (lipophilic) low molecular weight compounds (hydrocarbons, steroids, O2, N2, H2, CO2) occurs through free diffusion, which is governed by the first Fick's Law. These compounds usually penetrate a tiny pore that may form in the membrane for a short time (e.g. due to intense lipid movement). Small polar molecules such as water, urea or ethanol can also use the vacancies created by the chaotic and rapid movement of long chains to cross the membrane. The membrane is impermeable to hydrophilic substances; these substances can only pass through the membrane via various transporters or channels, e.g. water passes through channels called aquaporins.
Cooperativeness and flexibility[edit | edit source]
Another important property of the structure of biological membranes is cooperativity. It results from the repetitive application of non-covalent bonds. It has three important consequences: bilayers show a natural tendency to expand, close and harden (holes in the bilayer are energetically disadvantageous).
Flexibility refers to the ability of membranes to bend (form folds). This is another important property, it sets a lower limit of 25 nm for the size of vesicles that can form from the membrane.
Passive electrical properties of membranes[edit | edit source]
Membranes and direct current[edit | edit source]
If we plug a biological membrane into a DC circuit, it will, like most substances, exhibit the properties of a resistor (it will have resistance). The actual resistance of membranes will depend on many factors e.g. membrane composition or temperature. The specific electrical conductivity is different for membranes, for the extracellular and intracellular space. For membranes, the specific conductance can be around 10-⁶ -10-⁸ s/m, for the cytoplasm and intercellular space it is 0.2-1.0 s/m. When current passes through the membrane, depolarization of the membrane and an increase in its permeability also occurs.
Due to the high resistance of biological membranes, the DC current in the organism is mainly transmitted through the intercellular fluid.
Membranes and alternating current[edit | edit source]
Since the membrane is composed of two layers (plates) of phospholipids with a space (insulator) between them, it exhibits the characteristics of a capacitor when connected to an AC circuit. The membrane starts to form an electric field, is capable of accumulating electrical energy and has its own capacitance.
Therefore, if we use low frequencies for AC, the impedance of the membranes will be higher than if we use higher frequencies.
Links[edit | edit source]
Related articles[edit | edit source]
Použitá literatura[edit | edit source]
- BRUCE, Alberts – BRAY, D – JOHNSON, A. Základy buněčné biologie. 1. edition. Espero Publishing, 1998. 630 pp. ISBN 80-902906-0-4.
- AMLER, Evžen, Tomáš BLAŽEK a Jindřiška HEŘMANSKÁ. Praktické úlohy z biofyziky. [1. edition]. Praha: Ústav biofyziky 2. lékařské fakulty UK, 2006
- VAJNER, Luděk – UHLÍK, Jiří – KONRÁDOVÁ, Václava. Lékařská histologie I. Cytologie a obecná histologie. 1. edition. Karolinum, 2012. Chapter 1. pp. 8-10. ISBN 978-80-246-1860-9.
- VODRÁŽKA, Zdeněk. Biochemie. 2. edition. 1996. ISBN 8020006001.
- ZÁVODSKÁ, Radka. Biologie buněk. 1. edition. Scientia, 2006. Chapter 4. pp. 36. ISBN 80-86960-15-3.
- ŠVÍGLEROVÁ, Jitka. Biologická membrána [online]. ©Last revised 2009-02-18. [cit. 2010-11-12]. <https://web.archive.org/web/20160306065550/http://wiki.lfp-studium.cz/index.php/Biologická_membrána>.
- UHROVÁ, Helena. Elektrické pole v buňkách a v organismu [online]. [cit. 2015-10-25]. <http://fchi.vscht.cz/files/uzel/0010359/003Elektricke_pole_v_+bunkach_a_organismech.pdf>.